Summary of antimicrobial guidance
| Site: | Royal College of General Practitioners - Online Learning Environment |
| Course: | TARGET antibiotics toolkit hub |
| Book: | Summary of antimicrobial guidance |
| Printed by: | Guest user |
| Date: | Friday, 23 January 2026, 8:06 PM |
Summary of antimicrobial prescribing guidance - managing common infections
Version 1.2, September 2024
UKHSA, NICE and other collaborators are discussing options for continued production of the Summary of Antimicrobial Prescribing Guidance (previously hosted by BNF Publications). In the interim we have produced a list of the conditions in the table linked to available national guidance.
We and have also included a condensed version of the table summary that includes infections covered by NICE antimicrobial prescribing guidance.
Please refer to the user guide and principles of treatment when using the antimicrobial prescribing guidance summaries.
Fluoroquinolone antibiotics: In January 2024, the MHRA published a Drug Safety Update on fluoroquinolone antibiotics. These must now only be prescribed when other commonly recommended antibiotics are inappropriate. Stakeholders are assessing the impact of this warning on recommendations in the relevant guidance.
Please contact the TARGET team at TARGETantibiotics@UKHSA.gov.uk for additional information if required.
- Acne vulgaris – NICE
- Acute cough - NICE
- Acute otitis media - NICE
- Acute otitis externa – NICE/CKS
- Acute sore throat - NICE
- Bacterial vaginosis – BASHH
- Blepharitis – NICE/CKS
- Boils, carbuncles, and staphylococcal carriage - NICE/CKS
- Bronchiectasis (non-cystic fibrosis), acute exacerbation - NICE
- Candida – oral – NICE/CKS
- Cellulitis and erysipelas - NICE
- Chlamydia - BASHH
- Chickenpox – NICE/CKS
- Clostridioides difficile infection - NICE
- Conjunctivitis – NICE/CKS
- COPD (acute exacerbation) - NICE
- Coronavirus (COVID-19) - NICE
- Diabetic foot infection - NICE
- Diarrhoea – prevention and advice for travellers – NICE/CKS
- Diverticular disease - NICE
- Eczema - NICE
- Epididymitis - BASHH
- Fungal skin infection: body and groin – NICE/CKS
- Fungal skin infection: nail – NICE/CKS
- Gastroenteritis - NICE/CKS
- Gonorrhoea - BASHH
- Helicobacter pylori - NICE/BNF
- Herpes – genital - BASHH
- Herpes simplex – oral – NICE/CKS
- Human and animal bites - NICE
- Impetigo - NICE
- Influenza - UKHSA/CKS
- Insect bites and stings - NICE
- Leg ulcer - NICE
- Mastitis – NICE/CKS
- Pelvic inflammatory disease – BASHH
- Pneumonia (community-acquired) - NICE
- Pneumonia (hospital-acquired) - NICE
- Prostatitis (acute) - NICE
- PVL-SA - UKHSA (PHE)
- Pyelonephritis (acute) - NICE
- Scabies - BASHH
- Scarlet fever - NICE/CKS
- Shingles – NICE/CKS
- Sinusitis - NICE
- Suspected meningococcal disease – NICE/CKS
- Threadworm – NICE/CKS
- Tick bites (Lyme disease) - NICE
- Trichomoniasis - BASHH
- UTI (lower) - NICE
- UTI (recurrent) - NICE
- UTI (catheter-associated) - NICE
- Vaginal candidiasis - BASHH
Upper Respiratory Tract Infection text summaries
Please refer to the user guide and principles of treatment when using the antimicrobial prescribing guidance summaries.
Contents
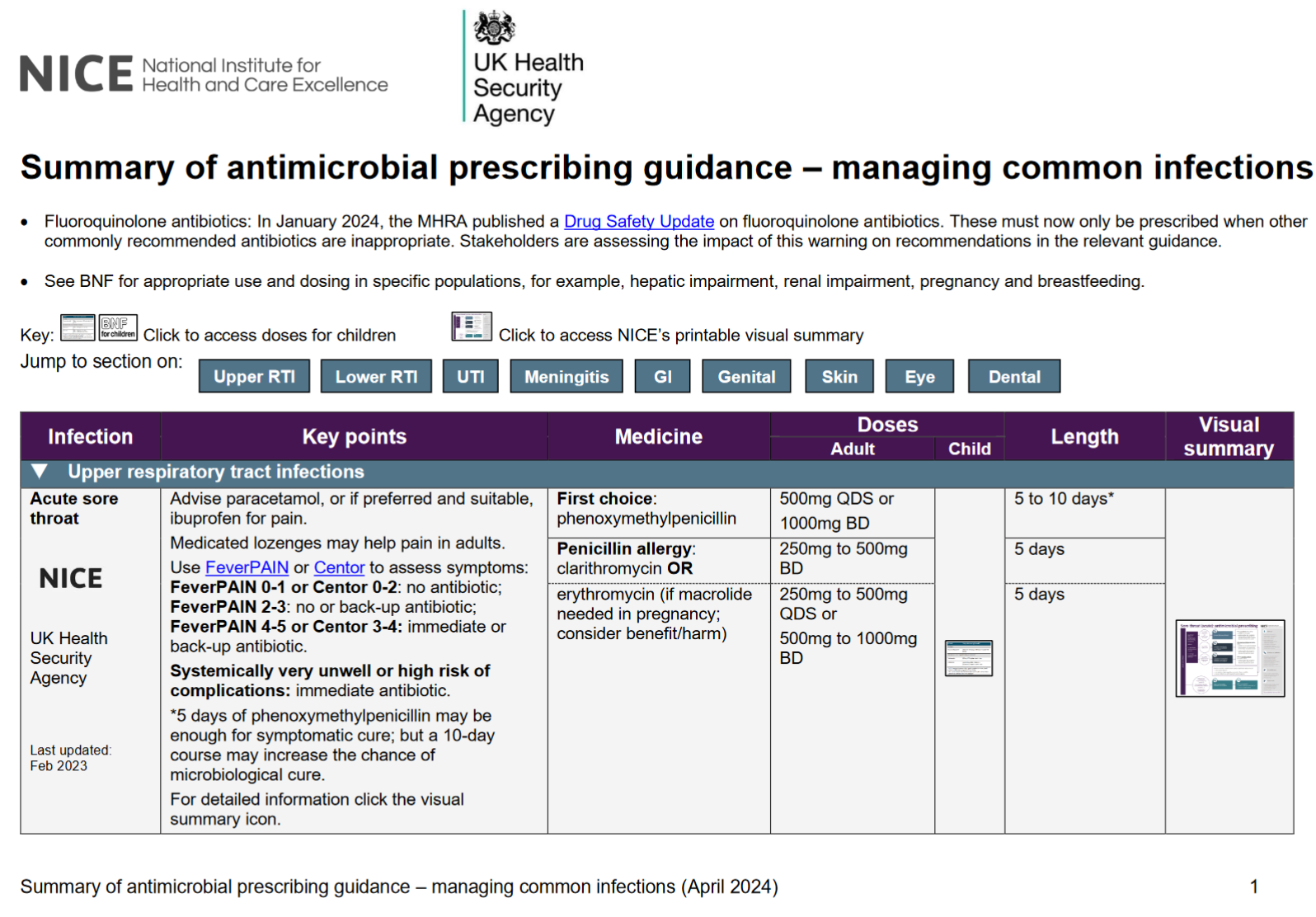
Acute Sore Throat
Advise paracetamol, or if preferred and suitable, ibuprofen for pain. Medicated lozenges may help pain in adults.
Use FeverPAIN or Centor to assess symptoms:
- FeverPAIN 0-1 or Centor 0-2: no antibiotic;
- FeverPAIN 2-3: no or back-up antibiotic;
- FeverPAIN 4-5 or Centor 3-4: immediate or back-up antibiotic.
Systemically very unwell or high risk of complications: immediate antibiotic.
5 days of phenoxymethylpenicillin may be enough for symptomatic cure; but a 10-daycourse may increase the chance of microbiological cure.
First choice adult:
phenoxymethylpenicillin 500mg QDS or 1000mg BD for 5 to 10 days*
Penicillin allergy adult:
clarithromycin 250mg to 500mg BD for 5 days
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 250mg to 500mg QDS or 500mg to 1000mg BD for 5 days
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng84
Last updated: Feb 2023
Acute Otitis Media
Regular paracetamol or ibuprofen for pain (right dose for age or weight at the right time and maximum doses for severe pain).
Consider ear drops containing an anaesthetic and an analgesic for pain if an immediate antibiotic is not given and there is no ear drum perforation or otorrhoea.
- Otorrhoea or under 2 years with infection in both ears: no, back-up or immediate antibiotic.
- Otherwise: no or back-up antibiotic.
- Systemically very unwell or high risk of complications: immediate antibiotic.
First choice adult:
amoxicillin for 5 to 7 days
Penicillin allergy adult:
clarithromycin for 5 to 7 days
OR
erythromycin (if macrolide needed in pregnancy: consider benefit/harm) for 5 to 7 days
Second choice adult:
co-amoxiclav for 5 to 7 days
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng91
Section last updated: Oct 2023Sinusitis
Advise paracetamol or ibuprofen for pain. Little evidence that nasal saline or nasal decongestants help, but people may want to try them.
- Symptoms for 10 days or less: no antibiotic.
- Symptoms with no improvement for more than 10 days: no antibiotic or back-up antibiotic depending on likelihood of bacterial cause. Consider high-dose nasal corticosteroid (if over 12 years).
- Systemically very unwell or high risk of complications: immediate antibiotic.
First choice adult:
phenoxymethylpenicillin 500mg QDS for 5 days
Penicillin allergy adult:
doxycycline (not in under 12s) 200mg on day 1, then 100mg OD for 5 days
OR
clarithromycin 500mg BD for 5 days
OR
erythromycin (if macrolide needed in pregnancy: consider benefit/harm) 250 to 500mg QDS or 500 to 1000mg BD for 5 days
Second choice or first choice if systemically very unwell or high risk of complications:
co-amoxiclav 500/125mg TDS for 5 days
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng79
Section last updated: Oct 2017
Lower Respiratory Tract Infection text summaries
Please refer to the user guide and principles of treatment when using the antimicrobial prescribing guidance summaries.
Contents
- COVID-19
- Acute Exacerbation of COPD
- Acute Exacerbation of Bronchiectasis (Non-Cystic Fibrosis)
- Acute Cough
- Hospital-Acquired Pneumonia
- Community-Acquired Pneumonia
COVID-19
Under Review (Refer to updated NICE guidance, Pneumonia: diagnosis and management, while we update the links in this summary)
Antibiotics should not be used for preventing or treating COVID-19 unless there is clinical suspicion of additional bacterial co-infection.
Do not use azithromycin to treat COVID-19.
Do not use doxycycline to treat COVID-19 in the community.
Do not offer an antibiotic for preventing secondary bacterial pneumonia in people with COVID-19.
If a person in the community has suspected or confirmed secondary bacterial pneumonia, start antibiotic treatment as soon as possible, see community-acquired pneumonia for choices.
In hospital, start empirical antibiotics if there is clinical suspicion of a secondary bacterial infection in people with COVID-19, see hospital-acquired pneumonia for choices. Start antibiotics as soon as possible after establishing a diagnosis of secondary bacterial pneumonia, and certainly within 4 hours. Start treatment within 1 hour if the person has suspected sepsis and meets any of the high-risk criteria for this outlined in the NICE guideline on sepsis.
For detailed information, see the NICE guideline on managing COVID-19.
Section last updated: December 2021
Acute Exacerbation of COPD
Many exacerbations are not caused by bacterial infections so will not respond to antibiotics.
Consider an antibiotic, but only after taking into account severity of symptoms (particularly sputum colour changes and increases in volume or thickness), need for hospitalisation, previous exacerbations, hospitalisations and risk of complications, previous sputum culture and susceptibility results, and risk of resistance with repeated courses.
Some people at risk of exacerbations may have antibiotics to keep at home as part of their exacerbation action plan.
First choice adult:
amoxicillin 500mg TDS (see BNF for severe infection) for 5 days
OR
doxycycline 200mg on day 1, then 100mg OD (see BNF for severe infection) for 5 days
OR
clarithromycin 500mg BD for 5 days
Second choice adult: use alternative first choice
Alternative choice adult (if person at higher risk of treatment failure):
co-amoxiclav 500/125mg TDS for 5 days
OR
co-trimoxazole 960mg BD for 5 days
OR
levofloxacin (only if other alternative choice antibiotics are unsuitable with specialist advice) 500mg OD for 5 days.
See the MHRA January 2024 advice on restrictions and precautions for using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects. Fluoroquinolones must now only be prescribed when other commonly recommended antibiotics are inappropriate.
IV antibiotics: see the guidance visual summary
For detailed information, see the guidance visual summary or https://www.nice.org.uk/guidance/ng115
Section last updated September 2024
Acute Exacerbation of Bronchiectasis (Non-Cystic Fibrosis)
Send a sputum sample for culture and susceptibility testing.
Offer an antibiotic.
When choosing an antibiotic, take account of severity of symptoms and risk of treatment failure. People who may be at higher risk of treatment failure include people who’ve had repeated courses of antibiotics, a previous sputum culture with resistant or atypical bacteria, or a higher risk of developing complications.
Course length is based on severity of bronchiectasis, exacerbation history, severity of exacerbation symptoms, previous culture and susceptibility results, and response to treatment.
Do not routinely offer antibiotic prophylaxis to prevent exacerbations.
Seek specialist advice for preventing exacerbations in people with repeated acute exacerbations. This may include a trial of antibiotic prophylaxis after a discussion of the possible benefits and harms, and the need for regular review.
First choice empirical treatment adult:
amoxicillin (preferred if pregnant) 500mg TDS for 7 to 14 days
OR
doxycycline (not in under 12s) 200mg on day 1, then 100mg OD for 7 to 14 days
OR
clarithromycin 500mg BD for 7 to 14 days
Alternative choice (if person is at higher risk of treatment failure) empirical treatment adult:
co-amoxiclav 500/125mg TDS for 7 to 14 days
OR
levofloxacin (adults only: with specialist advice if con-amoxiclav cannot be used: consider safety issues) 500mg OD or BD 7 to 14 days
OR
levofloxacin (adults only: only if con-amoxiclav is unsuitable; with specialist advice) 500mg OD or BD 7 to 14 days
See the MHRA January 2024 advice on restrictions and precautions for using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects. Fluoroquinolones must now only be prescribed when other commonly recommended antibiotics are inappropriate.
IV antibiotics: see the guidance visual summary
When current susceptibility data available: choose antibiotics accordingly
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng117/
Section last updated September 2024
Acute Cough
Some people may wish to try honey (in over 1s), the herbal medicine pelargonium (in over 12s), cough medicines containing the expectorant guaifenesin (in over 12s) or cough medicines containing cough suppressants, except codeine, (in over 12s). These self-care treatments have limited evidence for the relief of cough symptoms.
Acute cough with upper respiratory tract infection: no antibiotic.
Acute bronchitis: no routine antibiotic.
Acute cough and higher risk of complications (at face-to-face examination): immediate or back-up antibiotic.
Acute cough and systemically very unwell (at face-to-face examination): immediate antibiotic.
Higher risk of complications includes people with pre-existing comorbidity; young children born prematurely; people over 65 with 2 or more of, or over 80 with 1 or more of: hospitalisation in previous year, type 1 or 2 diabetes, history of congestive heart failure, current use of oral corticosteroids.
Do not offer a mucolytic, an oral or inhaled bronchodilator, or an oral or inhaled corticosteroid unless otherwise indicated.
First choice adults:
doxycycline 200mg day1, then 100mg OD for 5 days
Alternative first choice adults:
amoxicillin (preferred if pregnant) 500mg TDS for 5 days
OR
clarithromycin 250mg to 500mg BD for 5 days
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 250mg to 500mg QDS or 500mg to 1000mg BD for 5 days
First choice children:
amoxicillin for 5 days
Alternative first choice children:
clarithromycin for 5 days
OR
erythromycin for 5 days
OR
doxycycline (not in under 12s) for 5 days
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng120
Section last updated Feb 2019
Hospital-Acquired Pneumonia
Under Review (Refer to updated NICE guidance, Pneumonia: diagnosis and management, while this summary is reviewed)
If symptoms or signs of pneumonia start within 48 hours of hospital admission, see community acquired pneumonia.
Offer an antibiotic. Start treatment as soon as possible after diagnosis, within 4 hours (within 1 hour if sepsis suspected and person meets any high-risk criteria – see the NICE guideline on sepsis).
When choosing an antibiotic, take account of severity of symptoms or signs, number of days in hospital before onset of symptoms, risk of developing complications, local hospital and ward-based antimicrobial resistance data, recent antibiotic use and microbiological results, recent contact with a health or social care setting before current admission, and risk of adverse effects with broad spectrum antibiotics.
No validated severity assessment tools are available. Assess severity of symptoms or signs based on clinical judgement.
Higher risk of resistance includes relevant comorbidity (such as severe lung disease or immunosuppression), recent use of broad-spectrum antibiotics, colonisation with multi-drug resistant bacteria, and recent contact with health and social care settings before current admission.
If symptoms or signs of pneumonia start within days 3 to 5 of hospital admission in people not at higher risk of resistance, consider following community acquired pneumonia for choice of antibiotic.
First choice adults and children (non-severe and not higher risk of resistance):
co-amoxiclav for adults 500/125mg TDS for 5 days then review
co-amoxiclav for children see the guidance visual summary for children’s dosage
Alternative first choice adults (non-severe and not higher risk of resistance):
choice based on specialist microbiological advice and local resistance data
Options include:
doxycycline 200mg on day 1, then 100mg OD for 5 days then review
OR
cefalexin (caution in penicillin allergy) 500 mg BD or TDS (can increase to 1 to 1.5g TDS or QDS for 5 days then review
OR
co-trimoxazole 960mg BD for 5 days then review
OR
levofloxacin (only if switching from IV levofloxacin with specialist advice;) 500mg OD or BD for 5 days then review
See the MHRA January 2024 advice on restrictions and precautions for using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects. Fluoroquinolones must now only be prescribed when other commonly recommended antibiotics are inappropriate.
Children alternative first choice (non-severe and not higher risk of resistance): clarithromycin
other options may be suitable based on specialist microbiological advice and local resistance data
see the guidance visual summary for children’s dosage
For first choice IV antibiotics (severe or higher risk of resistance) and antibiotics to be added if suspected or confirmed MRSA infection see visual summary.
For detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng139
Section last updated September 2024
Community-Acquired Pneumonia
Under Review (Refer to updated NICE guidance, Pneumonia: diagnosis and management, while this summary is reviewed)
Assess severity in adults based on clinical judgement and guided by a mortality risk score (CRB65 or CURB65) when these scores can be calculated:
- low severity – CRB65 0 or CURB65 0 or 1
- moderate severity – CRB65 1 or 2 or CURB65 2
- high severity – CRB65 3 or 4 or CURB65 3 to 5.
1 point for each parameter: confusion, urea (>7 mmol/l), respiratory rate ≥30/min, low systolic (<90 mm Hg) or diastolic (≤60 mm Hg) blood pressure, age ≥65.
Assess severity in children based on clinical judgement.
Offer an antibiotic. Start treatment as soon as possible after diagnosis, within 4 hours (within 1 hour if sepsis suspected and person meets any high-risk criteria – see the NICE guideline on sepsis).
When choosing an antibiotic, take account of severity, risk of complications, local antimicrobial resistance and surveillance data, recent antibiotic use, and microbiological results.
First choice (low severity in adults or non-severe in children):
amoxicillin for adults 500mg TDS (higher doses can be used, see BNF) for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
amoxicillin for children see the guidance visual summary for age range and dosage
Alternative first choice (low severity in adults or non-severe in children):
doxycycline for adults 200mg on day 1, then 100mg OD for 5 days
Stop antibiotics after five days unless microbiological results suggest a longer course is needed or the person is not clinically stable
doxycycline for children (not in under 12s) see the guidance visual summary for age range and dosage
OR
clarithromycin 500mg BD for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
clarithromycin for children see the guidance visual summary for age range and dosage
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 500mg QDS for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) for children see the guidance visual summary for age range and dosage
First choice (moderate severity in adults):
amoxicillin 500mg TDS (higher doses can be used, see BNF) for 5 days
Stop antibiotics after five days unless microbiological results suggest a longer course is needed or the person is not clinically stable
With either (if atypical pathogens suspected)
clarithromycin 500mg BD for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 500mg QDS for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
Alternative first choice (moderate severity in adults):
doxycycline 200mg on day 1, then 100mg OD for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
OR
clarithromycin 500mg BD for 5 days
Stop antibiotics after five days unless microbiological results suggest a longer course is needed or the person is not clinically stable
First choice (high severity in adults or severe in children):
co-amoxiclav 500/125mg TDS for 5 days
Stop antibiotics after five days unless microbiological results suggest a longer course is needed or the person is not clinically stable
co-amoxiclav for children see the guidance visual summary for age range and dosage
With either (if atypical pathogens suspected)
clarithromycin 500mg BD for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
clarithromycin for children see the guidance visual summary for age range and dosage
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 500mg QDS for 5 days
Stop
antibiotics after five days unless microbiological results suggest a longer
course is needed or the person is not clinically stable
erythromycin for children (if macrolide needed in pregnancy; consider benefit/harm) see the guidance visual summary for age range and dosage
Alternative antibiotic if high severity, for penicillin allergy:
levofloxacin 500mg BD for 5 days. Stop antibiotics after five days unless microbiological results suggest a longer course is needed or the person is not clinically stable
See the MHRA January 2024 advice for restrictions and precautions on using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects.
IV antibiotics: check the guidance visual summary
For detailed information or for information on children’s dosage see the
guidance visual summary or see https://www.nice.org.uk/guidance/ng138/
Section last updated September 2024
Urinary Tract Infection text summaries
Please refer to the user guide and principles of treatment when using the antimicrobial prescribing guidance summaries.
Contents
- Lower Urinary Tract Infection
- Acute Pyelonephritis (Upper Urinary Tract)
- Acute Prostatitis
- Recurrent Urinary Tract Infections
- Catheter-Associated Urinary Tract Infection
Lower Urinary Tract Infection
Advise paracetamol or ibuprofen for pain.
Non-pregnant women: back up antibiotic (to use if no improvement in 48 hours or symptoms worsen at any time) or immediate antibiotic.
Pregnant women, men, children, or young people: immediate antibiotic.
When considering antibiotics, take account of severity of symptoms, risk of complications, previous urine culture and susceptibility results, previous antibiotic use which may have led to resistant bacteria and local antimicrobial resistance data.
If people have symptoms of pyelonephritis (such as fever) or a complicated UTI, see acute pyelonephritis (upper urinary tract infection) for antibiotic choices.
Non-pregnant adult women first choice:
nitrofurantoin (if eGFR ≥45 ml/minute) 100mg m/r BD (or if unavailable 50mg QDS) for 3 days
OR
trimethoprim (if low risk of resistance) 200mg BD for 3 days
Non-pregnant adult women second choice:
nitrofurantoin (if eGFR ≥45 ml/minute) 100mg m/r BD (or if unavailable 50mg QDS) for 3 days
OR
pivmecillinam (a penicillin) 400mg initial dose, then 200mg TDS for 3 days
OR
fosfomycin 3g single dose sachet single dose
Pregnant adult women first choice:
nitrofurantoin (avoid at term) – if eGFR ≥45 ml/minute 100mg m/r BD (or if unavailable 50mg QDS) for 7 days
Pregnant adult women second choice:
amoxicillin (only if culture results available and susceptible) 500mg TDS for 7 days
OR
cefalexin 500mg BD for 7 days
Treatment of asymptomatic bacteriuria in pregnant adult women:
choose from nitrofurantoin (avoid at term), amoxicillin or cefalexin based on recent culture and susceptibility results
Adult men first choice:
trimethoprim 200mg BD for 7 days
OR
nitrofurantoin (if eGFR ≥45 ml/minute) 100mg m/r BD (or if unavailable 50mg QDS) for 7 days
Adult men second choice:
consider alternative diagnoses basing antibiotic choice on recent culture and susceptibility results
Children and young people (3 months and over) first choice:
trimethoprim (if low risk of resistance)
OR
nitrofurantoin (if eGFR ≥45 ml/minute)
Children and young people (3 months and over) second choice:
nitrofurantoin (if eGFR ≥45 ml/minute and not used as first choice)
OR
amoxicillin (only if culture results available and susceptible)
OR
cefalexin
For information on children’s dosage see the guidance visual summary or https://www.nice.org.uk/guidance/ng138/.
For detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng138/.
See also the NICE guideline on urinary tract infection in under 16s: diagnosis and management and the UK Health Security Agency urinary tract infection: diagnostic tools for primary care.
Section last updated Oct 2018Acute Pyelonephritis (Upper Urinary Tract)
Advise paracetamol (+/- low-dose weak opioid) for pain for people over 12.
Offer an antibiotic.
When prescribing antibiotics, take account of severity of symptoms, risk of complications, previous urine culture and susceptibility results, previous antibiotic use which may have led to resistant bacteria and local antimicrobial resistance data.
Avoid antibiotics that don’t achieve adequate levels in renal tissue, such as nitrofurantoin.
Non-pregnant women and men first choice:
cefalexin 500mg BD or TDS (up to 1g to 1.5g TDS or QDS for severe infections) for 7 to 10 days
OR
co-amoxiclav (only if culture results available and susceptible) 500/125mg TDS for 7 to 10 days
OR
trimethoprim (only if culture results available and susceptible) 200mg BD for 14 days
OR
ciprofloxacin (only if other first choice antibiotics are unsuitable) 500mg BD for 7 days
Non-pregnant women and men IV antibiotics:
see the guidance visual summary
Pregnant women first choice:
cefalexin 500mg BD or TDS (up to 1g to 1.5g TDS or QDS for severe infections) for 7 to 10 days
Pregnant women second choice or IV antibiotics:
see the guidance visual summary
Children and young people (3 months and over) first choice:
cefalexin
OR
co-amoxiclav (only if culture results available and susceptible)
Children and young people (3 months and over) IV antibiotics: see the guidance visual summary
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng111/.
For detailed information click on the guidance visual summary. See also the NICE guideline on urinary tract infection in under 16s: diagnosis and management and the UK Health Security Agency urinary tract infection: diagnostic tools for primary care.
Section last updated September 2024
Acute Prostatitis
Advise paracetamol (+/- low-dose weak opioid) for pain, or ibuprofen if preferred and suitable.
Offer antibiotic.
Review antibiotic treatment after 14 days and either stop antibiotics or continue for a further 14 days if needed (based on assessment of history, symptoms, clinical examination, urine, and blood tests).
First choice adult (guided by susceptibilities when available):
ciprofloxacin 500mg BD for 14 days then review
See the MHRA January 2024 advice on restrictions and precautions for using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects.
OR
ofloxacin 200mg BD for 14 days then review
See the MHRA January 2024 advice on restrictions and precautions for using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects.
Alternative first choice if fluoroquinolone antibiotic is not appropriate (seek specialist advice; guided by susceptibilities when available):
trimethoprim (if fluoroquinolone not appropriate; seek specialist advice) 200mg BD for 14 days then review
Second choice adult (after discussion with specialist):
levofloxacin 500mg OD for 14 days then review
See the MHRA January 2024 advice on restrictions and precautions for using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects.
OR
co-trimoxazole 960mg BD for 14 days then review
IV antibiotics: see the guidance visual summary
For detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng110.
Section last updated September 2024
Recurrent Urinary Tract Infections
Refer or seek specialist advice for: men, trans-women and people with a male urinary system, people with recurrent upper UTI (rUTI), if the underlying cause of rUTI is unknown, pregnant people, children under 16 years (see NICE guideline on UTI in under 16s), people with suspected cancer (see NICE guideline on suspected cancer), and anyone who has had surgery structurally altering the urethra.
For non-pregnant women, trans men and non-binary people with a female urinary system:
- First advise about behavioural and personal hygiene measures and self-care treatments to reduce the risk of UTI (e.g. cranberry products etc…) (see NICE guideline on recurrent UTI).
- If no improvement after behavioural or personal hygiene measures or if these are not appropriate: for those who are experiencing perimenopause, menopause, or are postmenopausal, consider vaginal oestrogen (review within 12 months);
- If no improvement after vaginal oestrogen or if it is inappropriate: consider single-dose antibiotic prophylaxis for exposure to a trigger (review within 6 months);
- If no improvement after trying vaginal oestrogen, and/or prophylaxis for triggers and/or there is no identifiable trigger: consider methenamine hippurate as an alternative to daily prophylaxis, as long as any current UTI is treated (review within 6 months, and then every 12 months, or earlier if agreed with the person).
- If no improvement after antiseptic prophylaxis or if it is not appropriate: consider a trial of daily antibiotic prophylaxis (review within 6 months).
Antiseptic prophylaxis:
methenamine hippurate, 1g BD, review within 6 months and then every 12 months, or earlier if agreed with the person
First choice adult antibiotic prophylaxis:
trimethoprim (avoid in pregnancy) 200mg single dose when exposed to a trigger or 100mg nightly, review within 6 months
OR
nitrofurantoin (avoid at term) (if eGFR 45 ml/minute or more) 100mg single dose when exposed to a trigger or 50 to 100mg nightly, review within 6 months
Second choice adult antibiotic prophylaxis:
amoxicillin 500mg single dose when exposed to a trigger or 250mg nightly, review within 6 months
OR
cefalexin 500mg single dose when exposed to a trigger or 125mg nightly, review within 6 months.
For information on children’s dosage or for detailed information see the guidance visual summary or NICE.
For detailed information click on the visual summary. See also the NICE guideline on urinary tract infection in under 16s: diagnosis and management and the UK Health Security Agency urinary tract infection: diagnostic tools for primary care.
Section last updated April 2025
Catheter-Associated Urinary Tract Infection
Antibiotic treatment is not routinely needed for asymptomatic bacteriuria in people with a urinary catheter.
Consider removing or, if not possible, changing the catheter if it has been in place for more than 7 days. But do not delay antibiotic treatment.
Advise paracetamol for pain.
Advise drinking enough fluids to avoid dehydration.
Offer an antibiotic for a symptomatic infection. When prescribing antibiotics, take account of severity of symptoms, risk of complications, previous urine culture and susceptibility results, previous antibiotic use which may have led to resistant bacteria and local antimicrobial resistance data.
Do not routinely offer antibiotic prophylaxis to people with a short-term or long-term catheter.
Non-pregnant women and men first choice if no upper UTI symptoms:
nitrofurantoin (if eGFR ≥45 ml/minute) 100mg m/r BD (or if unavailable 50mg QDS) for 7 days
OR
trimethoprim (if low risk of resistance) 200mg BD for 7 days
OR
amoxicillin (only if culture results available and susceptible) 500mg TDS for 7 days
Non-pregnant women and men second choice if no upper UTI symptoms:
pivmecillinam (a penicillin) 400mg initial dose, then 200mg TDS for 7 days
Non-pregnant women and men first choice if upper UTI symptoms:
cefalexin 500mg BD or TDS (up to 1g to 1.5g TDS or QDS for severe infections) for 7 to 10 days
OR
co-amoxiclav (only if culture results available and susceptible) 500/125mg TDS for 7 to 10 days
OR
trimethoprim (only if culture results available and susceptible) 200mg BD for 14 days
OR
ciprofloxacin (only if other first-choice antibiotics are unsuitable) 500mg BD for 7 days
See the MHRA January 2024 advice for restrictions and precautions on using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects. Fluoroquinolones must now only be prescribed when other commonly recommended antibiotics are inappropriate.
Non-pregnant women and men IV antibiotics:
see the guidance visual summary
Pregnant women first choice:
cefalexin 500mg BD or TDS (up to 1g to 1.5g TDS or QDS for severe infections) for 7 to 10 days
Pregnant women second choice or IV antibiotics:
see the guidance visual summary
Children and young people (3 months and over) first choice:
trimethoprim (if low risk of resistance)
OR
amoxicillin (only if culture results available and susceptible)
OR
cefalexin
OR
co-amoxiclav (only if culture results available and susceptible)
Children and young people (3 months and over) IV antibiotics:
see the guidance visual summary
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng113
See also the UK Health Security Agency urinary tract infection: diagnostic tools for primary care.
Section last updated September 2024
Gastrointestinal Tract Infection text summaries
Please refer to the user guide and principles of treatment when using the antimicrobial prescribing guidance summaries.
Contents
Clostridioides difficile infection
For suspected or confirmed C. difficile infection, see UK Health Security Agency’s guidance on diagnosis and reporting.
Assess: whether it is a first or further episode, severity of infection, individual risk factors for complications or recurrence (such as age, frailty, or comorbidities).
Existing antibiotics: review and stop unless essential. If still essential, consider changing to one with a lower risk of C. difficile infection.
Review the need to continue: proton pump inhibitors, other medicines with gastrointestinal activity or adverse effects (such as laxatives), medicines that may cause problems if people are dehydrated (such as NSAIDs).
Do not offer antimotility medicines such as loperamide.
Offer an oral antibiotic to treat suspected or confirmed C. difficile infection.
For adults, consider seeking prompt specialist advice from a microbiologist or infectious diseases specialist before starting treatment.
For children and young people, treatment should be started by, or after advice from, a microbiologist, paediatric infectious diseases specialist or paediatric gastroenterologist.
If antibiotics have been started for suspected C. difficile infection, and subsequent stool sample tests do not confirm infection, consider stopping these antibiotics.
Adult first-line for first episode of mild, moderate, or severe:
vancomycin 125mg QDS for 10 days
Adult second-line for first episode of mild, moderate, or severe if vancomycin ineffective:
fidaxomicin 200mg BD for 10 days
For further episode in an adult within 12 weeks of symptom resolution (relapse):
fidaxomicin 200mg BD for 10 days
For further episode in an adult more than 12 weeks after symptom resolution (recurrence):
vancomycin 125mg QDS for 10 days
OR
fidaxomicin 200mg BD for 10 days
For alternative antibiotics if first- and second-line antibiotics are ineffective or for life-threatening infection: seek specialist advice (see the guidance visual summary)
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng199
Section last updated July 2021
Acute Diverticulitis
Acute diverticulitis and systemically well: Consider no antibiotics, offer simple analgesia (for example paracetamol), advise to re-present if symptoms persist or worsen.
Acute diverticulitis and systemically unwell, immunosuppressed, or significant comorbidity: offer an antibiotic.
Give oral antibiotics if person not referred to hospital for suspected complicated acute diverticulitis.
Give IV antibiotics if admitted to hospital with suspected or confirmed complicated acute diverticulitis (including diverticular abscess).
If CT-confirmed uncomplicated acute diverticulitis, review the need for antibiotics.
First-choice for adults (uncomplicated acute diverticulitis):
co-amoxiclav 500/125mg TDS for 5 days (a longer course may be needed based on clinical assessment)
Penicillin allergy for adults or if co-amoxiclav unsuitable:
cefalexin (caution in penicillin allergy) 500mg BD or TDS (up to 1g to 1.5g TDS or QDS for severe infections) AND metronidazole 400mg TDS for 5 days (a longer course may be needed based on clinical assessment)
OR
trimethoprim 200mg BD WITH metronidazole 400mg TDS for 5 days (a longer course may be needed based on clinical assessment)
OR
Ciprofloxacin (only if switching from IV ciprofloxacin with specialist advice) 500mg BD WITH metronidazole 400mg TDS for 5 days (a longer course may be needed based on clinical assessment)
See the MHRA January 2024 advice for restrictions and precautions on using fluoroquinolone antibiotics because of the risk of disabling and potentially long-lasting or irreversible side effects. Fluoroquinolones must now only be prescribed when other commonly recommended antibiotics are inappropriate
For IV antibiotics in complicated acute diverticulitis (including diverticular abscess): see visual summary or NICE guidance
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/NG147
Section last updated September 2024
Skin Infection text summaries
Please refer to the user guide and principles of treatment when using the antimicrobial prescribing guidance summaries.
Contents
Eczema (Bacterial Infection)
Manage underlying eczema and flares with treatments such as emollients and topical corticosteroids, whether antibiotics are given or not.
Symptoms and signs of secondary bacterial infection can include: weeping, pustules, crusts, no response to treatment, rapidly worsening eczema, fever and malaise.
Not all flares are caused by a bacterial infection, so will not respond to antibiotics.
Eczema is often colonised with bacteria but may not be clinically infected.
Do not routinely take a skin swab.
Not systemically unwell:
Do not routinely offer either a topical or oral antibiotic.
If an antibiotic is offered, when choosing between a topical or oral antibiotic, take account of patient preferences, extent and severity of symptoms or signs, possible adverse effects, and previous use of topical antibiotics because antimicrobial resistance can develop rapidly with extended or repeated use.
Systemically unwell:
Offer an oral antibiotic.
If there are symptoms or signs of cellulitis, see cellulitis and erysipelas.
If not systemically unwell, do not routinely offer either a topical or oral antibiotic
Topical antibiotic (if a topical is appropriate). For localised infections only:
First choice adult:
fusidic acid 2% TDS for 5 to 7 days
Oral antibiotic:
First choice adult:
flucloxacillin 500mg QDS for 5 to 7 days
Penicillin allergy or flucloxacillin unsuitable in adults:
clarithromycin 250mg BD (can be increased to 500mg BD for severe infections) for 5 to 7 days
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 250mg to 500mg QDS for 5 to 7 days
If MRSA suspected or confirmed – consult local microbiologist.
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng190
Section last updated March 2021Impetigo
Localised non-bullous impetigo:
Hydrogen peroxide 1% cream (other topical antiseptics are available but no evidence for impetigo).
If hydrogen peroxide unsuitable or ineffective, short-course topical antibiotic.
Widespread non-bullous impetigo:
Short-course topical or oral antibiotic.
Take account of person’s preferences, practicalities of administration, previous use of topical antibiotics because antimicrobial resistance can develop rapidly with extended or repeated use, and local antimicrobial resistance data.
Bullous impetigo, systemically unwell, or high risk of complications:
Short-course oral antibiotic.
Do not offer combination treatment with a topical and oral antibiotic to treat impetigo.
Topical antiseptic:
hydrogen peroxide 1% BD or TDS for 5 days
5 days treatment is appropriate for most, but can be increased to 7 days based on clinical judgement
Topical antibiotic:
First choice:
fusidic acid 2% TDS for 5 days
5 days treatment is appropriate for most, but can be increased to 7 days based on clinical judgement
Fusidic acid resistance suspected or confirmed:
mupirocin 2% TDS for 5 days
5 days treatment is appropriate for most, but can be increased to 7 days based on clinical judgement
Oral antibiotic:
First choice in adults:
flucloxacillin 500mg QDS for 5 days
5 days treatment is appropriate for most, but can be increased to 7 days based on clinical judgement
see guidance visual summary for child dosage
Penicillin allergy or flucloxacillin unsuitable in adults:
clarithromycin 250mg BD for 5 days
5 days treatment is appropriate for most, but can be increased to 7 days based on clinical judgement
see guidance visual summary for child dosage
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 250 to 500mg QDS for 5 days
5 days treatment is appropriate for most, but can be increased to 7 days based on clinical judgement
see guidance visual summary for child dosage
If MRSA suspected or confirmed – consult local microbiologist
For information on children’s dosage or for detailed information click the guidance visual summary or https://www.nice.org.uk/guidance/ng153
Section last updated February 2020
Insect Bites and Stings
Most insect bites or stings will not need antibiotics.
Do not offer an antibiotic if there are no symptoms or signs of infection.
If there are symptoms or signs of infection, see cellulitis and erysipelas.
For detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng182
Section last updated Oct 2023
Leg Ulcer Infection
Manage any underlying conditions to promote ulcer healing.
Only offer an antibiotic when there are symptoms or signs of infection (such as redness or swelling spreading beyond the ulcer, localised warmth, increased pain or fever). Few leg ulcers are clinically infected, but most are colonised by bacteria.
When prescribing antibiotics, take account of severity, risk of complications and previous antibiotic use.
First-choice in adults:
flucloxacillin 500mg to 1g QDS for 7 days
Penicillin allergy or if flucloxacillin unsuitable in adults:
doxycycline 200mg on day 1, then 100mg OD (can be increased to 200mg daily) for 7 days
OR
clarithromycin 500mg BD for 7 days
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 500mg QDS for 7 days
Second choice in adults:
co-amoxiclav 500/125mg TDS for 7 days
OR
co-trimoxazole (in penicillin allergy) 960mg BD for 7 days
For antibiotic choices if severely unwell or MRSA suspected or confirmed, click on the visual summary
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng152
Section last updated Feb 2020Cellulitis and Erysipelas
Exclude other causes of skin redness (inflammatory reactions or non-infectious causes).
Consider marking extent of infection with a single-use surgical marker pen.
Offer an antibiotic. Take account of severity, site of infection, risk of uncommon pathogens, any microbiological results and MRSA status.
Infection around eyes or nose is more concerning because of serious intracranial complications.
Do not routinely offer antibiotics to prevent recurrent cellulitis or erysipelas.
First choice:
Flucloxacillin (adults) 500mg to 1g QDS for 5 to 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
see the guidance visual summary for child dosage
Penicillin allergy or if flucloxacillin unsuitable:
clarithromycin (adults) 500mg BD for 5 to 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
see the guidance visual summary for child dosage
OR
erythromycin (adults) (if macrolide needed in pregnancy; consider benefit/harm) 500mg QDS 5 to 7 days
A longer course (up to 14 days
total) may be needed but skin takes time to return to normal, and full
resolution at 5 to 7 days is not expected
see the guidance visual summary for child dosage
OR
doxycycline (adults only) 200mg on day 1, then 100mg OD 5 to 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
OR
co-amoxiclav (children only: not in penicillin allergy) 5 to 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
see the guidance visual summary for child dosage
If infection near eyes or nose:
co-amoxiclav (adults) 500/125mg TDS for 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
see the guidance visual summary for child dosage
If infection near eyes or nose (penicillin allergy):
clarithromycin (adults) 500mg BD for 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
WITH
metronidazole (adults) (only add in children if anaerobes suspected) 400mg TDS for 7 days
A longer course (up to 14 days total) may be needed but skin takes time to return to normal, and full resolution at 5 to 7 days is not expected.
see the guidance visual summary for child dosage
For alternative choice antibiotics for severe infection, suspected or confirmed MRSA infection and IV antibiotics click on the visual summary. For information on children’s dosage or for detailed information click the visual summary icon.
Section last updated Sept 2019
Diabetic Foot Infection
In diabetes, all foot wounds are likely to be colonised with bacteria. Diabetic foot infection has at least 2 of: local swelling or induration; erythema; local tenderness or pain; local warmth; purulent discharge.
Severity is classified as:
- Mild: local infection with 0.5 to less than 2cm erythema
- Moderate: local infection with more than 2cm erythema or involving deeper structures (such as abscess, osteomyelitis, septic arthritis or fasciitis)
- Severe: local infection with signs of a systemic inflammatory response.
Start antibiotic treatment as soon as possible.
Take samples for microbiological testing before, or as close as possible to, the start of treatment.
When choosing an antibiotic, take account of severity, risk of complications, previous microbiological results and antibiotic use, and patient preference.
Do not offer antibiotics to prevent diabetic foot infection.
Mild infection: adult first choice
flucloxacillin 500mg to 1g QDS for 7 days*
Mild infection: adult penicillin allergy
clarithromycin 500mg BD for 7 days*
OR
erythromycin (if macrolide needed in pregnancy; consider benefit/harm) 500mg QDS for 7 days*
OR
doxycycline 200mg on day 1, then 100mg OD (can be increased to 200mg daily) for 7 days*
*A longer course (up to a further 7 days) may be needed based on clinical assessment. However, skin does take time to return to normal, and full resolution at 7 days is not expected.
For antibiotic choices for moderate or severe infection, infections where Pseudomonas aeruginosa or MRSA is suspected or confirmed, and IV antibiotics see the guidance visual summary.
For information on children’s dosage or for detailed information see the guidance visual summary or https://www.nice.org.uk/guidance/ng19/
Section last updated Oct 2019
Acne Vulgaris
First-line treatment options: offer a course of 1 of the options, taking account of severity, preferences, and advantages/disadvantages of each option. Completing the course is important because positive effects can take 6 to 8 weeks.
Consider topical benzoyl peroxide monotherapy as an alternative if first-line treatment options are contraindicated, or to avoid topical retinoids or an antibiotic (topical or oral).
Do not use: monotherapy with a topical antibiotic, monotherapy with an oral antibiotic, or a combination of a topical antibiotic and an oral antibiotic.
Review first-line treatment at 12 weeks.
Only continue a topical or oral antibiotic for more than 6 months in exceptional circumstances. Review at 3 monthly intervals and stop the antibiotic as soon as possible.
First line: for any acne severity, not in under 9s
fixed combination of topical adapalene with topical benzoyl peroxide
0.1% adapalene/ 2.5% benzoyl peroxide or 0.3% adapalene/2.5% benzoyl peroxide OD (thinly applied in the evening) for 12 weeks
OR
for any acne severity, not in under 12s
fixed combination of topical tretinoin with topical clindamycin
0.025% tretinoin/ 1% clindamycin OD (thinly applied in the evening) for 12 weeks
OR
for mild to moderate acne, not in under 12s
fixed combination of topical benzoyl peroxide with topical clindamycin
3% benzoyl peroxide/1% clindamycin or 5% benzoyl peroxide/1% clindamycin OD (thinly applied in the evening) for 12 weeks
OR
for moderate to severe acne, not in under 12s
fixed combination of topical adapalene with topical benzoyl peroxide AND either oral lymecycline or oral doxycycline
either 0.1% adapalene/ 2.5% benzoyl peroxide or 0.3% adapalene/2.5% benzoyl peroxide OD (thinly applied in the evening) AND either oral lymecycline 408mg or oral doxycycline 100mg OD for 12 weeks
OR
for moderate to severe acne, not in under 12s
topical azelaic acid AND either oral lymecycline or oral doxycycline
15% or 20% azelaic acid BD AND either lymecycline 408mg or doxycycline 100mg OD for 12 weeks
Alternative: topical benzoyl peroxide 5% benzoyl peroxide OD to BD for 12 weeks
For information on children’s dosage or for detailed information see the NICE guidance.
Section last updated June 2021
Human and Animal Bites
Offer an antibiotic for a human or animal bite if there are symptoms or signs of infection, such as increased pain, inflammation, fever, discharge or an unpleasant smell. Take a swab for microbiological testing if there is discharge (purulent or non-purulent) from the wound.
Do not offer antibiotic prophylaxis if a human or animal bite has not broken the skin.
Human bite: Offer antibiotic prophylaxis if the human bite has broken the skin and drawn blood.
Consider antibiotic prophylaxis if the human bite has broken the skin but not drawn blood if it is in a high-risk area or person at high risk.
Cat bite: Offer antibiotic prophylaxis if the cat bite has broken the skin and drawn blood.
Consider antibiotic prophylaxis if the cat bite has broken the skin but not drawn blood if the wound could be deep.
Dog or other traditional pet bite (excluding cat bite): Do not offer antibiotic prophylaxis if the bite has broken the skin but not drawn blood.
Offer antibiotic prophylaxis if the bite has broken the skin and drawn blood if it has caused considerable, deep tissue damage or is visibly contaminated (for example, with dirt or a tooth).
Consider antibiotic prophylaxis if the bite has broken the skin and drawn blood if it is in a high-risk area or person at high risk.
First choice:
co-amoxiclav (in adults) 250/125mg or 500/125mg TDS for 3 days for prophylaxis, 5 days for treatment*
see the visual summary for child dosage
Penicillin allergy or co-amoxiclav unsuitable (in those over 12 years):
doxycycline 200mg on day 1, then 100mg or 200mg daily for 3 days for prophylaxis, 5 days for treatment*
WITH
metronidazole 400mg TDS for 3 days for prophylaxis, 5 days for treatment*
seek specialist advice in pregnancy
see the visual summary for other management options and dosage in children
IV antibiotics: check visual summary
*course length can be increased to 7 days (with review) based on clinical assessment of the wound.
For information on children’s dosage or for detailed information click the visual summary icon.
Section last updated Oct 2023