_ RCGP Learning
Blog entry by _ RCGP Learning
Written by Dr Toni Hazell
It wasn’t what I wanted in my stocking as a child, but as a full-blown contraception nerd, I’m excited at the December 2025 launch of the new UK Medical Eligibility Criteria for contraceptive use (UKMEC)1. Published by the College of Sexual and Reproductive Healthcare (CoSRH), formerly the Faculty of Sexual and Reproductive Healthcare, the UKMEC is the gold-standard document on contraceptive safety.
Before getting into the changes, there are some important basics to remember:
- The UKMEC is about safety, not efficacy, although the document does include an efficacy table (figure 1).
- Methods are categorised from 1-4, as per figure 2. 1 and 4 are simple – no problem to use or absolutely contraindicated respectively; it’s in the 2s and 3s that your clinical judgment will be important.
- The UKMEC is one place where 2 + 2 ≠ 4. Two category 2s doesn’t automatically mean an absolute contraindication, but if they are both in the same area, it does signal a cumulative risk and the need for caution. More than one category 3 ‘may pose an unacceptable risk’.1
- Some methods have different numbers for initiation or continuation, reflecting the different risks attached to starting a method or continuing one that is already being used.
- If a condition isn’t covered in the UKMEC, that doesn’t necessarily mean that all contraception is safe for use. Consider seeking advice from secondary care, or, if you are a CoSRH member, submit an evidence request to their Clinical Effectiveness Unit and they will summarise the available evidence for you to use alongside your clinical judgment.2
- The UKMEC is intended to be applied only to contraceptive use. If a woman is getting an extra benefit from her method (for example the management of endometriosis), that may affect your risk/benefit calculation.
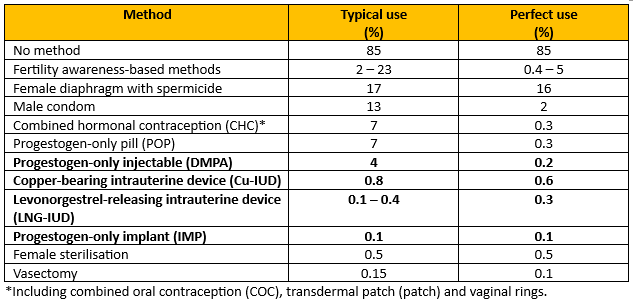
Figure 1 - Recreation of Percentage of women experiencing an unintended pregnancy within the first year of year of use with typical use and perfect use.
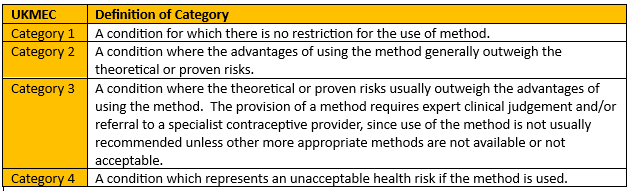
Figure 2 - Recreation of Definition of UKMEC categories
The key changes are summarised in figure 3.
|
Topic(s) |
Key change |
|
Chronic kidney disease. Multiple sclerosis.
|
|
|
Use of e-cigarettes. Sickle cell trait. |
|
|
Multiple category changes for the depot medroxyprogesterone acetate (DMPA) injection. |
|
|
Depression and anxiety |
|
|
Stroke |
|
|
Breast cancer |
|
|
Human papilloma virus and sexually transmitted infections. |
|
|
HIV |
|
|
Hypertension |
|
|
Multiple risk factors for VTE and CVD |
|
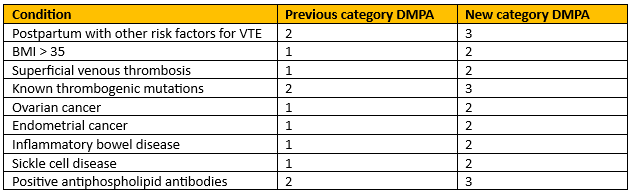
Figure 4 - Recreation of Conditions with increased risk of thrombosis.
Chronic Kidney Disease
Regarding CKD, only the most seriously affected are included – patients who either have nephrotic syndrome or are on dialysis. This cohort should not use CHC (due to VTE risk), and DMPA is now UKMEC 3. This is because DMPA is associated with a small loss in bone mineral density, reversible on stopping3 and those with chronic kidney disease (CKD) are already at risk of osteoporosis4. All other methods are a UKMEC 2.
Multiple sclerosis
The risk from MS is mainly to do with immobility as a risk factor for VTE, so most methods are a UKMEC 1 for those without prolonged immobility, the exception being DMPA, which is a 2, because those with MS have a greater risk of fracture than the greater population. With prolonged immobility, DMPA remains a 2 and CHC is a 3.
When prescribing hormonal contraception, it is common to be asked about whether it will cause mood changes. Mood alteration is listed as a common or very common side-effect in the BNF for some combined and progestogen only methods5,6,7 but depression was listed in the previous UKMEC as a category 1. It has been removed from this edition as a category and replaced with a statement about the effects of hormonal contraception in those with anxiety or mood disorders.
The key points are as follows8:
- There is no clear evidence that any form of hormonal contraception worsens or improves mood.
- Most evidence is from observational studies, which often have confounding factors, and do not usually focus on women with pre-existing mental health conditions.
- Some patients do report mood change during the use of hormonal contraception; this may not represent direct causation.
- Healthcare professionals should explore other possible contributing factors and consider alternative contraception if the patient feels that their mood has been adversely affected by their contraception.
- Patients with pre-existing anxiety or depression should monitor their mood when starting hormonal contraception.
There are two sections on multiple risk factors – one for CVD and one for VTE; the section on multiple risk factors for VTE has been updated in this iteration. The UKMEC signposts to NICE for a full list of risk factors but gives examples which include cancer, inflammatory disorders, recent trauma or surgery and being in the postnatal period. Someone with multiple risk factors for VTE is UKMEC 4 for CHC, 3 for DMPA and 1 for all other methods.
The UKMEC is a long document; it will take time for the changes to fully bed in, but practices will need to decide how they implement it, particularly for those already using contraception. Reviewing all those using DMPA at the time of their next injection, and everyone else at their annual review would be a good start and hopefully we will all be fully up to date with it long before the next one comes along in a decade or so!
References
- CoSRH. UK Medical Eligibility Criteria for Contraceptive Use (UKMEC). Dec 2025.
- CoSRH. Members’ Evidence Request Service.
- CoSRH. Progestogen-only Injectable Contraception. July 2023.
- National Osteoporosis Guideline Group UK. Clinical guideline for the prevention and treatment of osteoporosis. 2024.
- BNF. Ethinylestradiol with levonorgestrel.2025.
- BNF. Desogestrel.2025.
- BNF. Etonogestrel. 2025.