_ RCGP Learning
User blog: _ RCGP Learning
Written by Dr Toni Hazell
Stopping smoking is one of the most significant things that a person can do to improve their health. It is the second most cost-effective measure in COPD management (behind flu vaccination and ahead of all pharmaceuticals)1 and is associated with later mortality, whatever the age of quitting. It is however not an easy thing to do, with multiple studies showing that most people will relapse after their first quit event3,4,5 – encouragement to try again is therefore vital.
Smoking cessation is more likely to be successful with a combination of support and pharmacotherapy than when tried alone, or with just one of these modalities6. Our role as GPs is not usually to provide this support, but to signpost patients to stop smoking clinics and potentially to prescribe on the clinic’s behalf (depending on local commissioning arrangements). To do this effectively and safely, it helps to understand the principles of very brief advice (VBA), the basic biochemistry of smoking addiction and the pharmacology of the drugs currently available.
VBA is a NICE approved7 30-second intervention which can be delivered in a normal GP appointment; there is evidence that it is acceptable to patients and can contribute to successful smoking cessation8,9. VBA consists of three ‘As’ – ask, advise, act. Ask the patient if they smoke. Advise them how to stop smoking (by explaining that they are much more likely to succeed with support and pharmacotherapy than on their own) and advise them as to how they can access this support. This might be via referral, or by giving the patient details for the local self-referral scheme.
After attendance at a smoking cessation clinic, you may be asked to prescribe nicotine replacement therapy (NRT), or one of the three currently available drugs – varenicline, cytisinicline and bupropion7. These all act in different ways on the addiction mechanisms which keep people smoking. When a smoker inhales nicotine there is a rapid increase in dopamine10, causing an immediate sensation of pleasure; as nicotine and dopamine levels fall, the desire for the next cigarette increases. With long-term smoking, nicotine receptors are upregulated in both number and sensitivity11, causing dependence; down-regulation after quitting takes 6-12 weeks12. The table below gives brief information about what we can prescribe; more detail is available in our eLearning module, Essentials of Smoking Cessation, which also discusses e-cigarettes.
| Smoking cessation drug | Mechanism of action | Use in pregnancy, breastfeeding and under 18s | Other information (non-exhaustive list; consult BNF before prescribing) |
|---|---|---|---|
| NRT | Stimulates nicotine receptors to release dopamine, reducing withdrawal symptoms13. | Yes (from age 12). |
|
| Bupropion | Increases dopamine levels by reducing dopamine reuptake, reducing withdrawal symptoms14. | No |
|
| Varenicline | Stimulates nicotine receptors to release dopamine and blocks the rapid dopamine increase from nicotine16,18,19. | No |
|
| Cytisinicline | Stimulates nicotine receptors to release dopamine and blocks the rapid dopamine increase from nicotine16,18,19. | No |
|
References
- Murphy PB, Brueggenjuergen B, Reinhold T, et al. Cost-effectiveness of home non-invasive ventilation in patients with persistent hypercapnia after an acute exacerbation of COPD in the UK. Thorax 2023; 78: 523-525.
- Cho ER, Brill IK, Gram IT, et al. Smoking Cessation and Short- and Longer-Term Mortality. NEJM Evid. 2024 Mar; 3 (3): EVIDoa2300272.
- Gorniak B, Yong HH, Borland R et al. Do post-quitting experiences predict smoking relapse among former smokers in Australia and the United Kingdom? Findings from the International Tobacco Control Surveys. Drug Alcohol Rev. 2022 May; 41(4): 883-889.
- Lee SE, Kim CW, Im HB, et al. Patterns and predictors of smoking relapse among inpatient smoking intervention participants: a 1-year follow-up study in Korea. Epidemiol Health. 2021; 43: e2021043.
- Feuer Z, Michael J, Morton E, et al. Systematic review of smoking relapse rates among cancer survivors who quit at the time of cancer diagnosis. Cancer Epidemiol. 2022 Oct; 80: 102237.
- Stead LF, Koilpillai P, Fanshawe TR et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016 Mar 24; 3(3): CD008286.
- NICE. NG209. Tobacco: preventing uptake, promoting quitting and treating dependence. Feb 2025.
- Cheng CCW, He WJA, Gouda H, et al. Effectiveness of Very Brief Advice on Tobacco Cessation: A Systematic Review and Meta-Analysis. J Gen Intern Med. 2024 Jul; 39(9): 1721-1734.
- Papadakis S, Anastasaki M, Papadakaki M, et al. 'Very brief advice' (VBA) on smoking in family practice: a qualitative evaluation of the tobacco user's perspective. BMC Fam Pract. 2020 Jun 24; 21(1): 121.
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009; 49: 57-71.
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009 Oct 1; 78(7): 756-65.
- Cosgrove KP, Batis J, Bois F, et al. beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry. 2009 Jun; 66(6): 666-76.
- Molyneux A. Nicotine replacement therapy. BMJ. 2004 Feb 21; 328(7437): 454-6.
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005 Sep; 10(3): 219-31.
- Bupropion SPC. July 2024.
- Singh D, Saadabadi A. Varenicline. [Updated 2024 Oct 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-.
- Royal College of Psychiatrists. The prescribing of varenicline and vaping (electronic cigarettes) to patients with severe mental illness. Dec 2018.
- National centre for smoking cessation and training. Cytisine. 2024.
- Nides M, Rigotti NA, Benowitz N, et al. A Multicenter, Double-Blind, Randomized, Placebo-Controlled Phase 2b Trial of Cytisinicline in Adult Smokers (The ORCA-1 Trial). Nicotine Tob Res. 2021 Aug 29; 23(10): 1656-1663.
- NHS Wales. Cytisinicline. July 2024.
- Scottish Medicines Consortium. Medicines Advice.
- Stop Smoking NI.
- National Centre for Smoking Cessation and Training (NCSCT). 30 Seconds to change a life.
Written by Dr Dirk Pilat
It is part of our lives to be exposed to stressful events, none of us will be spared these. It is nevertheless human nature to adapt and adjust to these stressors and slowly recover. In general practice we regularly see people who seek solace, support or need help after distressing events in their life. These can be traumatic events, such as exposure to actual or threatened death, as well as nontraumatic events such as interpersonal conflict, unemployment, financial difficulties, or personal ill health. The majority of people suffering from these events don’t come to see us at all (the so called ‘symptom iceberg’), and those who see us often improve with empathetic and non-judgmental support, or benefit from appropriate signposting. Nevertheless, sometimes the stressful event can trigger a temporary episode that is more than just the normal reaction to distress. In this case it might be appropriate to consider the diagnosis of adjustment disorder.
The term ‘adjustment disorder’ has been around since 1980, and is usually associated with the following presentation, as suggested by DSM 5 – TR:
Emotional or behavioural symptoms within three months of a stressful event:
- exceeding what is acceptable within a cultural or societal context or
- associated with significant impairment within the social or occupational environment and
- does not meet the criteria for another significant mental health disorder and
- once the stressor is over, the symptoms do not persist.
Its prevalence is variable from country to country (particularly prevalent in those with current or recent conflict), and some papers suggest it is the seventh most common diagnostic category used by psychiatrists worldwide with a lifetime prevalence of up to 21%.
A meta-analysis from 2022 that aimed to determine those most at risk showed that female gender, unemployed status, low income, low social support, and a history of mental health disorders predicted adjustment disorders compared with no mental health condition. Those with adjustment disorder had 12 times the rate of suicide compared to those without this diagnosis.
Due to its presentation, adjustment disorder has a range of more severe differential diagnoses: for instance the diagnosis of PTSD and acute stress disorder require the stressful trigger to be of a magnitude that would be traumatic for almost everyone and also have a different group of symptoms. If the initial symptoms are worsening after a period of watchful waiting, the patient might be developing a severe mental disorder such as depression or general anxiety, particularly if the stressor vanishes.
The diagnosis is often criticised as the medicalisation of problems of modern life, as it may pathologise a normal stress response. We nevertheless know in primary care that different individuals will develop different responses to the same stressors (as the COVID-19 pandemic so powerfully demonstrated): some people will have no issues, some people will develop minor symptoms and handle them easily, while some people will develop an adjustment disorder. The decision whether the symptoms of our patient represents an adaptive or abnormal response is therefore a clinical one: some authors suggest assessing whether there is functional impairment present, whether the trajectory of the patient’s symptoms shows adaptation and whether the trajectory demonstrates the patient's resilience.
If the decision is made that support from primary care will not suffice, the next treatment of choice for people with adjustment disorders is psychological therapy, so signposting to your local talking therapies team (or a referral, if necessary) would be an appropriate next step. If significant distress and/or insomnia is present, short term pharmacological management with benzodiazepines might be appropriate in extreme cases. As the condition by definition is self-resolving, the use of medication is controversial, and has the potential to cause harm through side effects or dependence. Some papers suggest the use of a sedative anti-depressant such as trazodone may be helpful but there are no universal recommendations. There are randomised, placebo controlled trials that have shown the benefits of herbal remedies such as valerian or kava-kava (though there have been reports about hepatotoxicity). When recommending herbal supplements, always suggest that the patient checks the product for the THR Certification Mark: this indicates that the herbal medicine has been registered with the Medicines and Healthcare products Regulatory Agency and meets the required standards relating to its quality, safety, evidence of traditional use.
Placed in the middle of the community and with intricate knowledge of our patients, primary care is well placed to support patients with adjustment disorders, as we are able to assess the severity of their symptoms over time and are aware of their social circumstances, coping skills, beliefs and attitudes. This will help us determine whether the period of distress is settling or whether is causing a more severe mental health problem.
References
- Bachem R, Casey P. Adjustment disorder: A diagnosis whose time has come. Journal of Affective Disorders. 2018 Feb; 227: 243–53.
- Casey P, Bailey S. Adjustment disorders: the state of the art. World Psychiatry [Internet]. 2011 Feb; 10(1): 11–8.
- Geer K. Adjustment Disorder. Primary Care: Clinics in Office Practice. 2023 Mar;50(1):83–8.
- Gradus J. Prevalence and prognosis of stress disorders: a review of the epidemiologic literature. Clinical Epidemiology [Internet]. 2017 May; Volume 9: 251–60.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Kava Kava. [Updated 2018 Apr 10].
- McAteer A, Elliott AM, Hannaford PC. Ascertaining the size of the symptom iceberg in a UK-wide community-based survey. British Journal of General Practice. 2011 Jan 1; 61(582): e1–11.
- Medicines and Healthcare products Regulatory Agency: The Traditional Herbal Registration (THR) Certification Mark: Guidance for Business.
Written by Dr Toni Hazell
An ectopic pregnancy is one in which the fertilised egg implants outside of the uterus; around 95% are in a fallopian tube, but an ectopic can also occur on the ovary, elsewhere in the pelvis, and sometimes in the cervix or in a caesarean section scar. Around 1% of all pregnancies are ectopic, with this figure at least doubling for pregnancies arising from in-vitro fertilisation (IVF)1,2. Non-tubal ectopic pregnancies are associated with higher morbidity and mortality than tubal ectopics, often presenting late with a sudden onset of symptoms prior to rupture, collapse and death.
Bleeding from an ectopic pregnancy can be fatal and the numbers of deaths in the UK and Ireland increased between the 2018-20 and 2020-22 reporting periods (8 deaths in 2018-20 and 12 in 2022-22)3,4, with the 2024 MBRRACE-UK report noting that there were learning points for both primary and secondary care and that improvements to care may have made a difference for nine out of the 12 women who died. None were judged to have received good care, with three cases where improvements would probably not have affected the outcome. Many of these were around the presentation of women who did not know that they were pregnant; the rest of this blog will focus on the lessons for general practice. Those wanting more information on lessons for secondary care or the ambulance service can find them in the 2024 MBRRACE-UK report4.
Always consider the possibility of pregnancy
Unless there is a clear history of a total hysterectomy and bilateral oophorectomy, always consider pregnancy in a woman of reproductive age. Women who have irregular periods, or who have bled at the time of implantation, may not realise that they have missed a period; those who are pregnant as a result of failed contraception might not consider it as a possibility. Pregnancies due to a failure of sterilisation or intrauterine contraception in particular are at higher risk for being ectopic.
Symptoms may be atypical
When presented with a woman who has pelvic pain and/or vaginal bleeding, with a delayed period, most of us would consider an ectopic pregnancy. This may not be the case if the presentation is more subtle. Symptoms can include breast tenderness, diarrhoea or vomiting, dizziness, shoulder tip pain and rectal pressure, and some women will collapse with no previous symptoms and no knowledge that they are pregnant1,4. NICE gives the following advice: “During clinical assessment of women of reproductive age, be aware that they may be pregnant, and think about offering a pregnancy test even when symptoms are non-specific”5. If a home pregnancy test was done, consider whether it may have been done too early, such that a false negative result was obtained.
Do you know where pregnancy tests are kept at work?
General practice is not resourced to offer routine testing for confirmation of pregnancy, and this has in some cases led to tests being unavailable or locked away. It is important that all practices hold some easily accessible pregnancy tests so that they can be used when there is a clinical reason to do so. Women may not have done a test at home because they didn’t think pregnancy was a possibility, or because of issues with access or affordability; adolescents in particular are less likely to confirm pregnancy promptly with a test6.
There are issues with access to early pregnancy assessment units (EPAUs)
The MBRRACE-UK report4 described calls not returned, issues with language barriers, and waits of over 24 hours for an appointment. Clearly any woman who is haemodynamically unstable at presentation should be referred via the emergency department, rather than via EPAU, but a woman who is stable when seen by her GP, may not remain so over the course of a long wait for an EPAU appointment. Commissioning GPs reading this blog might want to reflect on EPAU access in their area; are women reliably seen within 24 hours, seven days a week, and does the service have access to enough people to answer the phone, and interpretation available in person and on the phone? As GPs, we should not be reticent in saying if we feel that a service for an individual patient isn’t good enough, and if an EPAU appointment isn’t offered in a reasonable timeframe then referral to the on-call gynaecology team may be appropriate.
Health inequalities matter
Vulnerable women are over-represented among those who died from an ectopic in 2020-2022, with one-sixth having language difficulties and nearly half having either a mental health diagnosis or issues with substance use or domestic abuse. These inequalities may prevent women’s concerns from being heard; practices should consider how the care of women who may find it more difficult to get their message across can be optimised, even in the difficult, time-pressured situation of the current NHS. The RCGP’s Health Inequalities Hub has more resources in this area.
How do you manage a pregnant woman with a past ectopic, or who is at a high risk for ectopic pregnancy?
Almost one in five women who has had an ectopic pregnancy will have another one1; this is largely unaffected by the mode of treatment for the original ectopic. Aftercare of a woman who has had an ectopic should include the fact that she needs a scan at around 6-7 weeks of gestation in any subsequent pregnancies, to ensure that the pregnancy is intrauterine1. Depending on local pathways, this may need to be arranged by the GP, or the woman may be given open access to EPAU to arrange this. An early scan should also be arranged if there is a history of damage to the fallopian tubes (including in pregnancy after failed or reversed sterilisation7), and in women who become pregnant with an intrauterine device in place; up to 50% of such pregnancies may be ectopic.
Further information
The MBRRACE-UK report emphasises the need to Think Ectopic, which aligns with a national campaign led by The Ectopic Pregnancy Trust, endorsed by the RCGP. At the heart of the campaign is an ectopic pregnancy biocard which reminds healthcare professionals about the signs of ectopic pregnancy and time critical next steps.
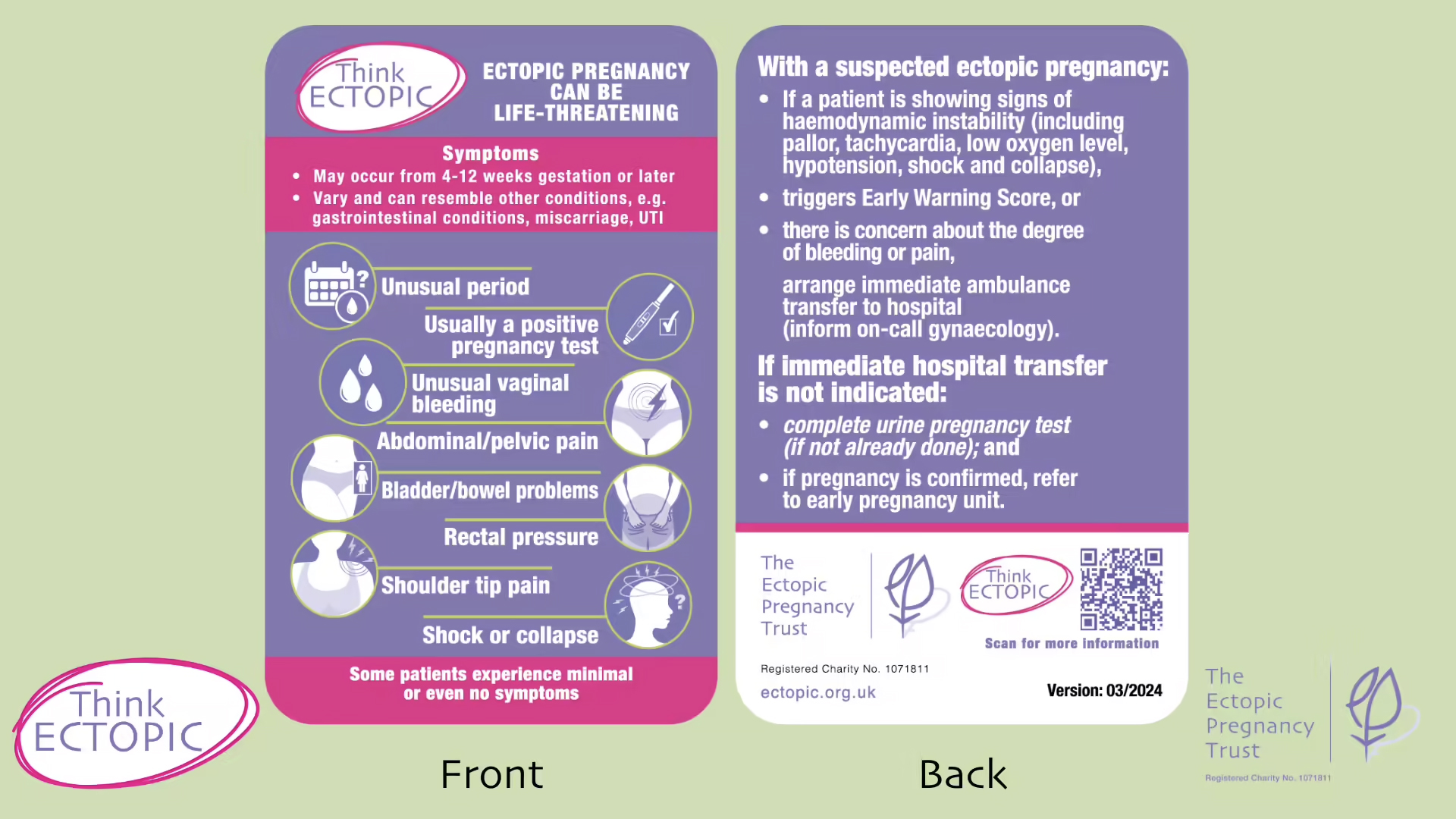
Figure: Think Ectopic Bio Card. The Ectopic Pregnancy Trust. Reproduced with permission.
Request free copies of the card from The Ectopic Pregnancy Trust.
References
- NICE CKS. Ectopic pregnancy. Feb 2023.
- Anzhel S, Mäkinen S, Tinkanen H et al. Top-quality embryo transfer is associated with lower odds of ectopic pregnancy. Acta Obstet Gynecol Scand. 2022 Jul; 101(7): 779-786.
- MBRRACE-UK. Saving Lives, Improving Mothers’ Care. Nov 2022.
- MBRRACE-UK. Saving Lives, Improving Mothers’ Care. Oct 2024.
- NICE. NG126. Ectopic pregnancy and miscarriage: diagnosis and initial management. Aug 2023.
- Ralph LJ, Foster DG, Barar R, et al. Home pregnancy test use and timing of pregnancy confirmation among people seeking health care. Contraception. 2022 Mar; 107: 10-16.
- University Hospitals Coventry and Warwickshire NHS Trust. Laparoscopic sterilisation. Dec 2022.
- FSRH. Intrauterine contraception. July 2023.
Written by Dr Toni Hazell
Please note: this document was last updated on April 15 2024
1. There are treatments available for community management of COVID-19
For most of the pandemic, the primary care management of those with COVID-19 was supportive – if the patients weren’t unwell enough to be admitted then we managed them in the same way as patients with other viral respiratory tract infections. This changed in early 2022 with the arrival of new community treatments, which can be divided into two groups – antivirals and neutralising monoclonal antibodies (nMABs).
NICE1 has approved two drugs for use in the community. The first is nirmatrelvir plus ritonavir (an antiviral, given orally) and it is approved for adults who do not need supplemental oxygen for COVID-19 and have an increased risk for progression to severe COVID-19. The second option is sotrovimab (an nMAB, given intravenously), which is approved for adults and children who are at least 12 and weigh at least 40kg, for whom nirmatrelvir plus ritonavir is contraindicated or unsuitable. They also have to have no need for supplemental oxygen and be in the group with an increased risk for progression to severe COVID-19.
2. Only the highest risk patients are eligible for these treatments
Patients who are a member of the ‘highest risk group’ should have all received a letter from NHSEI telling them about these new treatments. They were also initially sent a PCR test kit to keep at home so that they can test promptly if they have symptoms of COVID-19. Advice has now changed and they are advised to take a lateral flow test as soon as possible if they have any symptoms that could be COVID-192. If positive, this should be uploaded online.
The group of patients defined as being in the ‘highest risk group’ is given in the table below – it is not identical to the group who were asked to shield during the pandemic, nor is it the same as the group who are usually invited for flu vaccinations.
The following patient cohorts were determined by an independent advisory group commissioned by the Department of Health and Social Care (DHSC)1.
|
Down's syndrome and other genetic disorders |
|
|
Solid cancer |
|
|
Haematological diseases and recipients of haematological stem cell transplant (HSCT) |
|
|
Renal disease |
|
|
Liver diseases |
|
|
Solid organ transplant recipients |
Solid organ transplant recipients not in any of the above categories. Immune-mediated inflammatory disorders (diseases in which autoimmune or autoinflammation-based pathways are implicated in disease, for example, inflammatory arthritis, connective tissue diseases, inflammatory skin diseases, inflammatory gastrointestinal disease).
|
|
Respiratory |
|
|
Immune deficiencies |
|
|
HIV/AIDS |
|
|
Neurological disorders |
|
|
Children and young people (CYP) at substantial risk |
Complex life-limiting neurodisability with recurrent respiratory infections or compromise. |
|
CYP at significant risk if 2 or more of these risk factors are present |
Primary immunodeficiency:
Secondary immunodeficiency:
Immunosuppressive treatment:
Other conditions:
|
Table 1: Patient cohorts considered at highest risk from COVID-19 and to be prioritised for treatment with nMABs. Nirmatrelvir plus ritonavir, sotrovimab and tocilizumab for treating COVID-19. National Institute for Health and Care Excellence (NICE) Technology Appraisal (TA), 878, March 2023, last updated: 13 March 2024.
3. Commissioning of these treatments in England has changed.
When these treatments were first offered they were centrally commissioned - a high-risk patient in England who uploaded a positive COVID-19 test would be contacted directly by a COVID-19 medicine delivery unit (CMDU). Commissioning is now done locally4 and patients need to make contact themselves. Depending on local arrangements, they may do this via the GP, via their hospital consultant, via 111 or by contacting the CMDU directly. As time passes, it may be that some areas choose to use a mechanism other than a CMDU to deliver care. It would be sensible to find out how care is offered in your area, before you need to signpost a patient to it. The provision of these medications is arranged differently in Scotland5, Wales6, and Northern Ireland7.
In April 2024, the BMA issued guidance in this area, in response to suggestions from some ICBs that COVID-19 therapeutics should routinely be given by GPs. The BMAs position is that provision of therapeutics for COVID-19 should not be done by primary care, unless this is part of an appropriately commissioned service9.
4. Some patients may not realise that they are at high risk.
COVID-19 and the shielding programme revealed some of the limitations of coding and it is likely that there will be patients in the highest risk group who are unaware of their risk, particularly if they have only recently acquired the condition which makes them high risk. If you speak to such a patient then you can let them know that they are at high risk and should have some COVID-19 tests at home, and how to access treatment in your area.
5. Referring to a CMDU should be easy
If you have a patient who you think needs to be seen at a CMDU then you can refer directly, via e-RS. Your local CMDU should be found within the infectious diseases menu. At the moment, none of these drugs can be prescribed by a GP or by any doctor outside of a CMDU and they are not stocked by community pharmacies. Some of them have significant drug interactions, so if referring from primary care it is important to provide a list of current medication.
6. The PANORAMIC trial8 is now closed
The Platform Adaptive trial of NOvel antiviRals for eArly treatMent of Covid-19 in the Community (PANORAMIC) was open to those with COVID-19 who did not fit the criteria for treatment from a CMDU but had a wider range of co-morbidities or were aged over 50. It offered usual care or usual care plus a COVID-19 therapeutic. The group eligible for this trial were more similar to the group who are routinely offered flu vaccination, than to the shielding group. The trial is now closed to recruitment but will continue to collect data until September 2024. It is hoped that the date from PANORAMIC will inform our understanding about longer-term COVID-19 symptoms, as well as the use of antivirals, as participants will be contacted for six months after their enrolment in the trial.
References
- NICE Technology Appraisal. Nirmatrelvir plus ritonavir, sotrovimab and tocilizumab for treating COVID-19. March 2023, last updated March 2024.
- Department of Health and Social Care and UK Health Security Agency. COVID-19: guidance for people whose immune system means they are at higher risk. Last updated May 2024.
- Department of Health and Social Care. Independent report. Defining the highest risk clinical subgroups upon community infection with SARS-CoV-2 when considering the use of neutralising monoclonal antibodies (nMABs) and antiviral drugs. Last updated March 2023.
- Powis S. Access to COVID treatments. NHS England, June 2023.
- NHS Inform. Coronavirus (COVID-19): Treatments. May 2023.
- Antiviral services across Wales – information for members of the public. July 2023.
- Nidirect Government Services. Treatments for coronavirus (COVID-19).
- PANORAMIC trial.
- BMA. Guidance on Covid Therapeutics for GPs.
Written by Dr Emma Nash
Phosphate homeostasis
Phosphate is routinely reported as part of a bone profile blood test. Bone profiles are commonly used to assess calcium and alkaline phosphatase levels, but abnormal phosphate – usually low – is a frequent incidental finding.
Inorganic phosphate is measured as serum phosphate. It has many functions in the body, including calcium homeostasis, cell membrane structure (as phospholipids), DNA and RNA structure, bone growth and mineralisation, intracellular energy control, neuromuscular function, and extracellular pH maintenance.1
85% of phosphate stores are in bone, mainly as hydroxyapatite. Absorbed phosphorous is converted into its inorganic form by alkaline phosphatase and enters the extracellular fluid pool. From here it moves into bones and tissues as needed. Homeostatic control of phosphate is complex, and occurs through the intestine-bone-kidney-parathyroid axis. A summary of key components, and their effects, are shown in table 1. Some of the mechanisms work to both increase and decrease phosphate levels, depending on the site of action. Intestinal absorption, renal calcitriol (1,25-dihydroxycholecalciferol - the active form of vitamin D) and parathyroid hormone (PTH) are the greatest regulators of serum phosphate levels, although oestrogens, glucocorticoids, metabolic acidosis and various growth factors (particularly fibroblast growth factor 23 – FGF23) are also involved.2
|
Table 1: Control of phosphate homeostasis1,2,3 |
||
|
Substance |
Action |
Net effect on phosphate levels |
|
Calcitriol. |
Increases phosphate (and calcium) absorption from gut. Stimulates FGF23 production. Increases phosphate (and calcium) reabsorption in renal tubules. Inhibits PTH production. |
↑ |
|
PTH. |
Increases renal phosphate excretion (increases calcium reabsorption). Stimulates calcitriol synthesis. Stimulates FGF23 production, promoting bone turnover and calcium and phosphate release. |
↓ |
|
FGF23 |
Promotes bone turnover leading to phosphate release. Inhibits PTH secretion. Reduces renal phosphate reabsorption. Reduces calcitriol secretion. |
↓ |
Intestinal absorption
Phosphorous is present in a wide variety of foods, in both organic and inorganic forms, with the latter more easily absorbed. Intestinal absorption predominantly takes place in the duodenum and jejunum, with around 60-70% of intake being absorbed. Dietary intake from animal sources is greater than from plants. Dairy and bakery products make up the majority of our dietary intake. Phosphate additives are also commonly found in processed foods as preservatives and stabilisers. The widespread presence of phosphate in dietary sources means that a healthy adult with an ‘average’ diet should not develop dietary deficiency.4
Normal phosphate levels 
Local laboratory reference ranges may vary, but normal levels in children aged 1-16 are usually 1.3-2.4 mmol/L, and 0.8-1.5mmol/L in adults. It is important to note that serum levels may naturally be reduced by having had a recent meal (due to insulin production), in the winter due to lower vitamin D, in elderly men, and first thing in the morning. Paraproteinaemia can also cause a spurious low phosphate.5 Phosphate levels do not change significantly in pregnancy, but are higher in lactating women, infants and children. It is worth considering these factors when receiving an abnormal result, and some areas prefer a fasting phosphate test.6
Causes of hypophosphataemia
Medication
- Chronic use of antacids: these antacids form a complex with dietary phosphate, preventing its absorption. This is more prominent with those containing aluminium hydroxide, but those containing calcium carbonate can also decrease intestinal absorption of dietary phosphorous. Regular use of three months or more would normally be required to result in an intestinal deficiency, although this may be sooner in those on a low-phosphate diet (e.g. with advanced chronic kidney disease). Persistent use of magnesium-containing salts can also reduce phosphate absorption.2
- Thiazide diuretics: studies have found that hypophosphataemia is more common in patients taking thiazide (but not loop) diuretics, particularly in patients with hypokalaemia and/or a raised BMI.8
- Glucocorticoids and mineralocorticoids.
Intestinal causes
- Alcohol excess: reduced dietary intake, impaired absorption, increased urinary excretion.
- Severe malnutrition and refeeding syndrome: the shift from a catabolic state to an anabolic state can unmask a whole-body phosphate deficiency, even if the serum phosphate is normal prior to refeeding. This can produce a marked reduction in phosphate levels. This is most common in anorexia nervosa, but can also occur where malnutrition has arisen through medical problems such as cancer, alcohol excess and conditions where chewing/swallowing are severely impaired.4
- Chronic diarrhoea.
Intra/extracellular shift
- Following treated diabetic ketoacidosis (DKA): the shift in glucose following DKA treatment can result in hypophosphataemia.
- Rapid uptake of phosphate: this can occur in acute leukaemia, but more often is seen following parathyroidectomy for primary hyperparathyroidism. The sudden drop in PTH results in an influx of calcium and phosphate into bones. It can persist for several months following surgery, and is informally known as ‘hungry bone syndrome’.7
- Hyperinsulinaemia.
Other metabolic causes
- Hypothyroidism.
- Hypokalaemia.
- Hyperparathyroidism (primary and secondary).
- Vitamin D deficiency.
- Hypercalcaemia.
Consequences and management

Mild hypophosphataemia (0.6-0.8 mmol/L) is usually asymptomatic. Chronic hypophosphataemia below 0.6 mmol/L may still be asymptomatic, but muscle weakness, myalgia, bone pain and changes to mental status (e.g. confusion) can be seen. At this level, phosphate replacement is warranted, ensuring that the person also has adequate calcium and vitamin D stores. The dose is 4-6 effervescent tablets per day, although a lower dosage may be needed if diarrhoea occurs.9 Replacement should usually be adequate after 7-10 days.10 Alongside replacement, assess for potential causes, recognising that phosphate replacement can affect the PTH-calcium-vitamin D axis, so investigative bloods may need to be done early.
Symptomatic hypophosphataemia usually occurs when phosphate levels are below 0.32 mmol/L,11 and if this occurs acutely, intravenous replacement may be indicated. Severe hypophosphataemia can cause multi-organ cellular dysfunction and impaired energy metabolism. Examples of life-threatening consequences include acute respiratory failure, acute heart failure, arrhythmias, rhabdomyolysis, encephalopathy and seizures.
Mild to moderately low levels do not need urgent input, and time can be taken to ascertain whether they are pathologically low or confounded by any of the factors already mentioned. Repeating the test fasting may be useful, along with considering any recent hospital admissions (severe illness, diabetic ketoacidosis and parathyroidectomy can all result in hypophosphataemia), and reviewing any regular medications to see if they may be contributing to the findings. If not already available, consider checking calcium, urea and electrolytes, vitamin D, PTH and thyroid function tests.
If otherwise asymptomatic and not severe, simple monitoring is all that is needed.5
References
1. RL Wadsworth, S Siddiqui, Phosphate homeostasis in critical care, BJA Education, Volume 16, Issue 9, September 2016, Pages 305–309.
2. Goretti Penido M, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012 Nov;27(11):2039-2048.
3. Ureña Torres PA, De Brauwere DP. Three feedback loops precisely regulating serum phosphate concentration. Kidney International. 2011; Volume 80, Issue 5, 443 – 445.
4. National Institutes of Health. Phosphorous: Fact Sheet for Health Professionals. 2023. [accessed 29 November 2024].
5. Glendenning P, Bell D, Clifton-Bligh R. Investigating hypophosphataemia. BMJ 2014;348:g3172.
6. Exeter Clinical Laboratory International. Phosphate. 2019. [accessed 29 November 2024].
7. Witteveen JE, van Thiel S, Romijn JA et al. THERAPY OF ENDOCRINE DISEASE: Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. European Journal of Endocrinology. 2013; Volume 168, Issue 3 Pages R45–R53.
8. Bosman A, Campos-Obando N, de Keyser C et al. Diuretic Use and Serum Phosphate: Rotterdam Study and UK Biobank. Journal of the Endocrine Society. 2024; Volume 8, Issue 5, bvae057.
9. British National Formulary. Phosphate. 2024. [accessed 29 November 2024].
10. Pappoe LK, Singh A. Hypophosphatemia. Editor(s): Stuart B. Mushlin, Harry L. Greene. Decision Making in Medicine (Third Edition). Mosby. 2010. Pages 392-393.
11. Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med 2005; 118: 1094–101.
Written by Dr Dirk Pilat
Introduction
Mpox (previously known as monkeypox) is a viral, infectious disease caused by a strain of the monkeypox virus (MPXV). The first human cases were identified in 1970 in the rainforest areas of Central and West Africa. Until 2022, outbreaks were usually limited to this geographical area, with occasional imported cases into non-epidemic countries and human to human transmission only present in a limited number of cases.
MPXV has two distinct genetic lineages, called clade 1 and 2, both split into types a and b. The 2022-2023 global outbreak of mpox that continues to this day is due to clade 2b and caused 3,822 cases in the UK over the last two years.
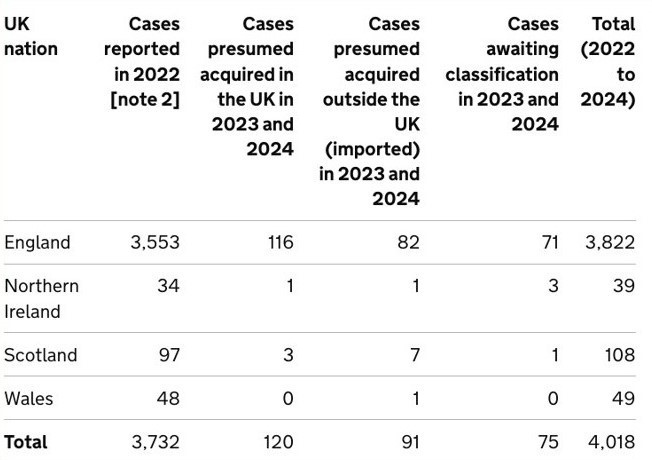
The 2022-23 outbreak was driven by human to human MPXV transmission via close contact with infected individuals and continues to this day, albeit in lower levels and particularly among men who have sex with men. In 2024, clade 1b mpox cases were starting to spread outside its traditional geographical location in West Africa with the Democratic Republic of the Congo, Republic of the Congo, Central African Republic, Burundi, Rwanda, Uganda, Kenya, Cameroon and Gabon all affected and around 20,000 cases and potentially 1000 deaths reported in the Democratic Republic of the Congo alone. On 14th August 2024 the World Health Organization declared a public health emergency over mpox and since then two imported cases have been identified in Sweden and Thailand.
Transmission
Mpox is transmitted through skin to skin contact, inhalation or contact with mucous membranes, through direct contact with skin lesions, coughing and sneezing and contact with clothing or linen used by person with mpox. Sexual contact, kissing, cuddling or other skin-to-skin contact can all cause transmission.
Signs and symptoms
While short incubation periods have been reported, a contact tracing study from the United Kingdom showed that 95% of people with a potential infection had symptoms within 16-23 days. There have been reports that clade 1b might have a shorter incubation record, but there remains a significant degree of uncertainty. Typical prodrome of the illness are fever, headache, myalgia and joint pain and lymphadenopathy, but proctitis and sore throats have also been reported. In the 2022-23 outbreak the subsequent rash was usually limited to fewer than 20 lesions, but there is anecdotal evidence that the rash associated with clade 1b is more severe with up to several thousand pustules. Similarly, mortality seems to be increased in the new outbreak, though this might be due to the affected population being more vulnerable. The rash initially presents with macules, before developing into papules, vesicles, pseudo-pustules containing solid debris, crusts, and finally scabs, before falling off within 7–14 days. These can be painful and are often located orally or in the genital or anorectal areas.
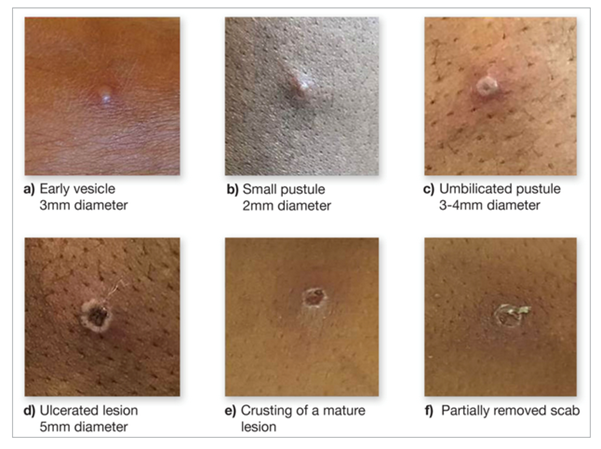
Mpox lesions in various stages of progression. UKHSA. Contains public sector information licensed under the Open Government Licence v3.0.
Management
Making a diagnosis of mpox can be difficult, as there is a broad range of differential diagnoses for pyrexial maculopapular viral rashes such as chickenpox, measles, bacterial skin infections, scabies and herpes. A travel, contact and sexual history is important to narrow the possible diagnosis down.
If triaging a patient with suspected mpox or if a patient presents with suspected mpox in general practice, the UK Health Security Agency (UKHSA) suggests isolating the patient in a single room and contacting the local infection service for advice, including appropriate arrangements for transfer into secondary care and immediate precautions in the setting (and who will manage contract tracing and advise on vaccination). When examining a suspected patient, clinical staff should wear face fit tested FFP3 masks, eye protection, long-sleeved splash resistant gowns and gloves to provide care if immediately required.
Treatment is usually symptomatic, but there are antivirals available in secondary care for those with severe disease or immunocompromised patients. The UKHSA has released guidance for pre- and post-exposure vaccination with the smallpox vaccine for patients, contacts and healthcare workers (see references).
References
Africa Centres for Disease Control and Prevention (Africa CDC). Africa CDC Epidemic Intelligence Weekly Report, August 2024. [accessed 2 September 2024].
Branda F, Romano C, Ciccozzi M, et al. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. J Clin Med. 2024 Apr 12;13(8):2234.
Centers for Disease Control and Prevention (CDC). Clinical Recognition: Key Characteristics for Identifying Mpox. Aug 2023. [accessed 2 September 2024].
Center for Health Security Johns Hopkins University. Situation Update Mpox Virus: Clade I and Clade IIb. 2024. [accessed 2 September 2024].
European Centre for Disease Prevention and Control (ECDC). Factsheet for health professionals on mpox. Aug 2024. [accessed 2 September 2024].
Reardon S. Mpox is spreading rapidly. Here are the questions researchers are racing to answer. Nature. 2024 Sep;633(8028):16-17.
UK Health Security Agency (UKHSA). Clade I mpox virus infection. Aug 2024. [accessed 2 September 2024].
UK Health Security Agency (UKHSA). Mpox: background information. Aug 2024. [accessed 2 September 2024].
UK Health Security Agency (UKHSA). Recommendations for the use of pre-and post-exposure vaccination during a monkeypox incident. Aug 2022. [accessed 2 September 2024].
World Health Organization (WHO). Mpox. Aug 2024. [accessed 2 September 2024].
Written by Dr Dirk Pilat
Topiramate is a sulfamate-substituted monosaccharide discovered in 1979 and first sold in 1996 as an anticonvulsant. In the United Kingdom it is licensed as monotherapy of generalised tonic-clonic seizures or focal seizures with or without secondary generalisation, adjunctive treatment of generalised tonic-clonic seizures or focal seizures with or without secondary generalisation, adjunctive treatment for seizures associated with Lennox-Gastaut syndrome and for migraine prophylaxis (British National Formulary, 2024). In other countries it is used off-label for alcohol use disorders and in combination with phentermine it is licensed as an aid to weight loss in the United States (Fariba & Saadabadi, 2024). It is well absorbed from the gastrointestinal tract with peak plasma levels usually achieved in 2—3 hours. It interacts with other anticonvulsants and decreases the efficacy of hormonal contraceptives and doses should be reduced in patients with severely impaired renal function, as it is predominately cleared through the kidneys (Spritzer, 2016).
After concerns around topiramate’s potential to cause teratogenic effects in pregnant women, the United Kingdom’s Medicines and Healthcare Products Agency (MHRA) asked the Commission on Human Medicines to look at findings from studies examining the risks associated with the use of topiramate during pregnancy. This showed that children born to mothers on topiramate during their pregnancy had a 2-3 times higher risk of intellectual disability, autism spectrum disorders (ASD) and attention deficit hyperactivity disorder (ADHD). There is also increased risk of congenital malformations and low birth weight. Prior to the review, topiramate was already known to be associated with significant harm during pregnancy, including a higher risk of birth defects and low birth weight (Medicines and Healthcare products Regulatory Agency, 2024).
To minimise risk for affected patients, the MHRA has introduced a Pregnancy Prevention Programme. It has released guidance for healthcare practitioners with patients suffering from migraine or epilepsy for both clinicians and patients. Topiramate is now contraindicated in pregnancy and in women of childbearing potential unless the conditions of a Pregnancy Prevention Programme are fulfilled. When initiated for epilepsy, the specialist prescriber team should counsel the patient accordingly based on the conditions of the MHRA’s healthcare professional guide, with primary care to
- Ensure continuous use of highly effective contraception in all women of childbearing potential.
- Check that all patients on the topiramate Pregnancy Prevention Programme have an up to date, signed, Annual Risk Awareness Form when a repeat prescription is issued.
- Remind all female patients that they will need to see their specialist at least once every year while taking topiramate.
- Refer to the specialist urgently (within days) in case of unplanned pregnancy and inform the patient not to stop taking topiramate until told to do so by her specialist.
- Refer any female patient planning to become pregnant to the specialist. Inform her not to stop using contraception or topiramate until told to do so by her specialist.
If topiramate would be initiated for migraine prophylaxis in primary care, the MHRA suggest that prescribers should:
- Assess potential for pregnancy and, if necessary, discuss the need for the patient to be on the Pregnancy Prevention Programme if she is to take topiramate.
- Discuss the risks with the patient and ensure they understand the:
- other therapeutic options available
- risks to the unborn child if topiramate is taken during pregnancy
- the need to take highly effective contraception throughout treatment and undergo pregnancy testing when required – e.g. if there is any reason to suggest lack of compliance or effectiveness of contraception
- the need to contact her primary care team urgently if they suspect they might be pregnant or wish to plan a pregnancy.
- Before the first prescription is issued in women of childbearing potential:
- exclude pregnancy (by serum pregnancy test)
- arrange for highly effective contraception.
- Complete the Annual Risk Awareness Form with the patient and give them a copy.
- Provide a copy of the Patient Guide to the patient.
- See the patient promptly in case of unplanned pregnancy or if she wants to plan a pregnancy.
All patients on the Pregnancy Prevention Programme should be invited for an annual review and only continue if its conditions are fulfilled. Patients should have an up to date and signed annual risk awareness form. Women should be using at least one highly effective method of contraception, preferably a LARC method. For those on migraine prophylaxis, women planning a pregnancy should stop topiramate at least a month before trying to conceive. For patients with epilepsy planning to become pregnant should be referred to their specialist and told not to stop using contraception or topiramate until told to do so by her specialist. Patients with unplanned pregnancies on topiramate should be referred urgently (Medicines and Healthcare products Regulatory Agency, 2024) (Medicines and Healthcare products Regulatory Agency, 2024).
To implement this into day to day primary care, a member of the team can:
- do a search on female patients on topiramate
- invite patients to discuss the pregnancy prevention programme
- for those on topiramate for epilepsy, write to the local neurologists to notify them of the patient and request a review by the specialist prescribing team
- discuss alternatives for those on topiramate for migraine
- counsel re the benefits of LARC solutions
- initiate or check current contraceptive solution
- audit female patients on topiramate six months after the implementation of the primary prevention programme to check whether there are gaps in the provision.
Resources
Topiramate Pregnancy Prevention
Healthcare Professional Guide:
For Epilepsy
For Migraine
Topiramate patient card
MHRA documents:
Topiramate: review of safety in pregnancy; Public Assessment Report
Announcement of new safety measures for topiramate
Direct Healthcare Professional Communication by Janssen-Cilag
References
Fariba KA, Saadabadi A. Topiramate. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–.
Medicines and Healthcare products Regulatory Agency. Topiramate Healthcare Professional Guide - Epilepsy. Jul 2024. [accessed 11 July 2024].
Medicines and Healthcare products Regulatory Agency. Healthcare Professional Guide Prophylaxis of Migraine. Jul 2024. [accessed 11 July 2024].
Medicines and Healthcare products Regulatory Agency. Topiramate: review of safety in pregnancy. Public Assessment Report. Jun 2024. [accessed 11 July 2024].
NICE. Topiramate. [accessed 11 July 2024].
Spritzer SD, Bravo TP, Drazkowski JF. Topiramate for Treatment in Patients With Migraine and Epilepsy. Headache. 2016 Jun;56(6):1081-5.
Written by Dr Toni Hazell
Public health authorities have noted a recent rise in both measles and pertussis cases. The first three months of 2024 saw 2,793 laboratory-confirmed cases of pertussis in England (compared to 858 in the whole of 2023), with so far five known deaths, all in infants1. There have been fewer cases of measles (826 over the same time frame2), but again this is a significant increase when compared to the 212 cases seen in the last quarter of 20232.
Prior to the introduction of vaccination, both of these infections were endemic. There were 800,000 cases of measles per year in the UK3, with 120,000 cases of pertussis per year in England and Wales4. Infections and deaths fell when vaccination was introduced, but the success of vaccinations for both infections has been hindered by reduced uptake5, with pertussis also affected by immunity that wanes over time. This was illustrated by an increase in cases in 2011/12, despite sustained levels of vaccine coverage over 95%6.
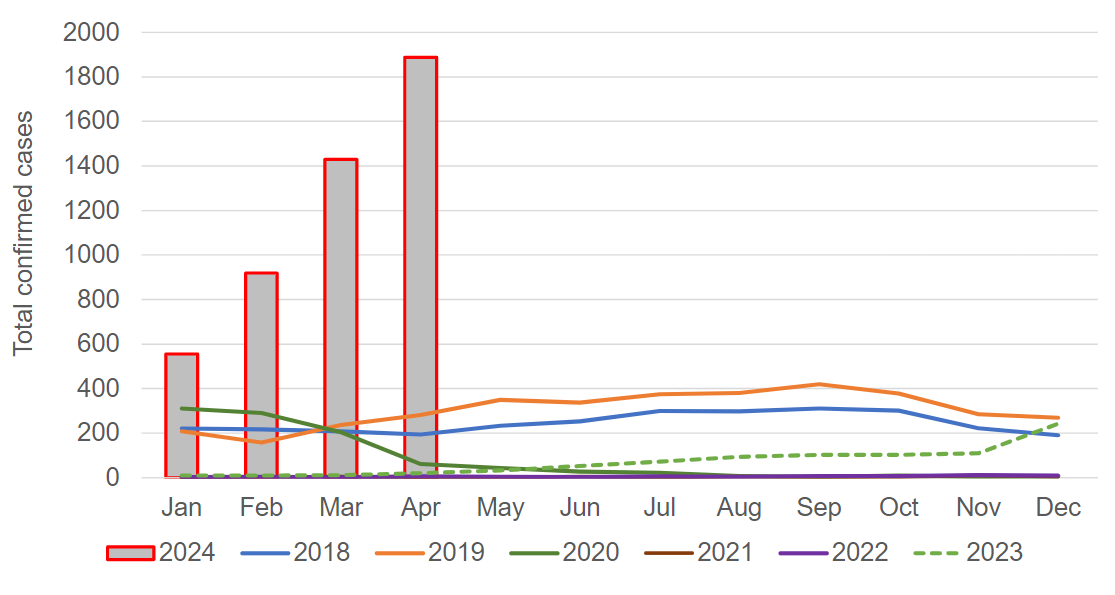
Laboratory confirmed cases of pertussis in England in 2024, compared to previous years1.
Image used under the Open Government v3.0 licence.
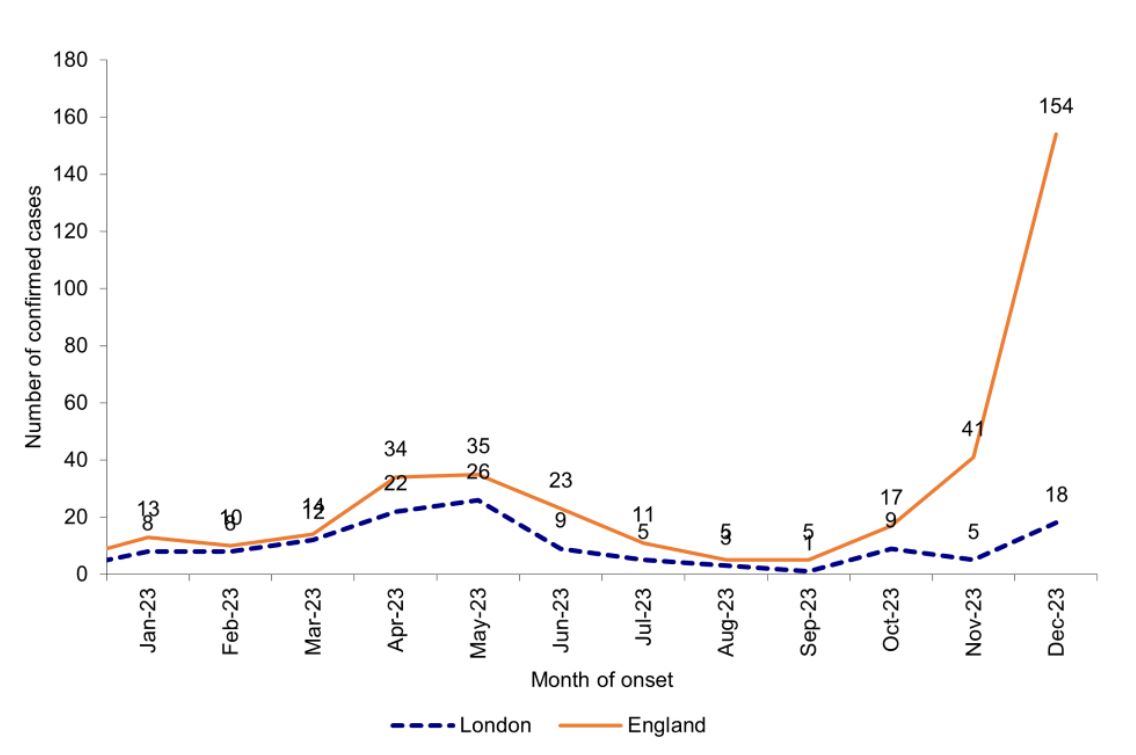
Laboratory confirmed cases of measles by month of onset of rash or symptoms reported7.
Image used under the Open Government v3.0 licence.
GPs working at the moment will not have been in clinical
practice when these infections were endemic, and so may need a reminder of how
to spot them – clinical features are outlined below. Both infections
present with a prodrome which is similar to a usual viral upper respiratory
infection; diagnosis at this point is probably less likely than when specific
features develop later on in the evolution of the illness.
Both illnesses have the potential for unpleasant complications. Patients who have had measles are more susceptible to opportunistic infection for around three years after their recovery, because the measles virus suppresses the immune response. Common complications include otitis media, diarrhoea and pneumonia. Rarer, but potentially more severe, are convulsions, encephalitis and subacute sclerosing panencephalitis. This last condition occurs on average seven years after the measles infection (but has been known up to 30 years later) – it presents with neurological symptoms and is always fatal3. Complications of pertussis include pneumonia, apnoea, seizures and encephalopathy. The strength of the cough can cause pneumothorax, abdominal hernia, rib fracture and subconjunctival haemorrhage. It is particularly risky in newborns – anyone aged under six months with suspected pertussis should be referred to paediatrics and is likely to be admitted4.
- Prodrome of upper respiratory tract symptoms and conjunctivitis, which starts 10-12 days after contracting the infection and lasts for 2-4 days, at which point a rash appears.
- Children generally feel unwell, with many references describing them as ‘miserable’.
- Fever, which peaks at around 39°C, coinciding with appearance of the rash.
- Rash with the following features:
- Starts on the face and behind the ears, before spreading down the body and finally to the extremities.
- Becomes confluent as it spreads.
- Erythematous and maculopapular.
- Lasts in total around one week, fading in any given area around five days after first appearance.
- May be less prominent (or not appear at all) in those who are immunosuppressed.
- Koplik spots on the buccal mucosa:
- 2-3mm across.
- Red, with white centres.
- Pathognomic for measles, but their absence does not exclude the condition.
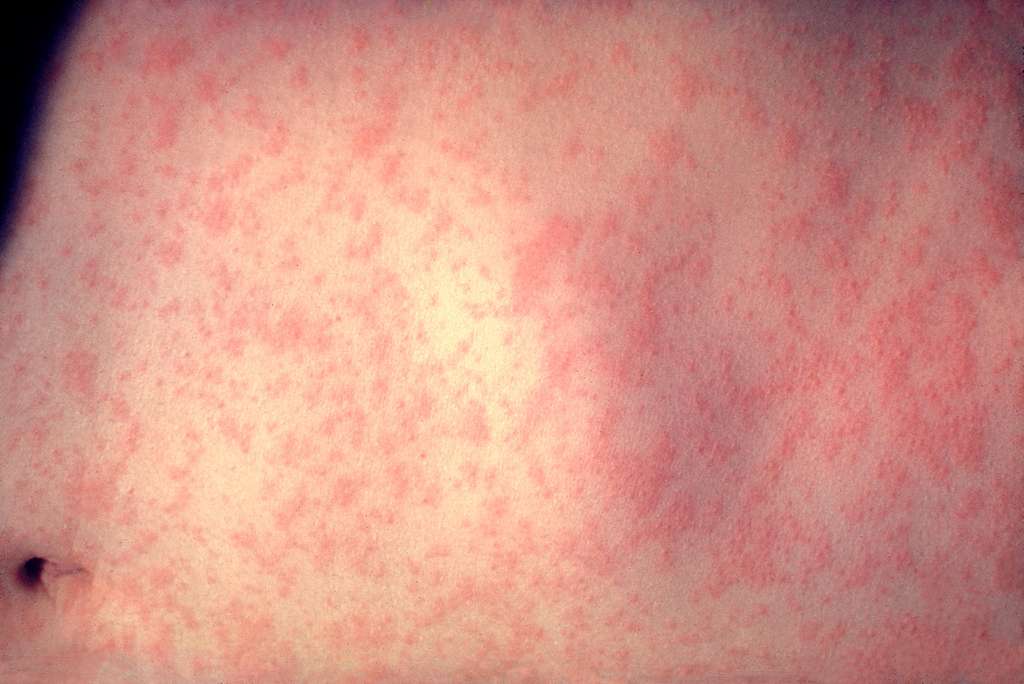
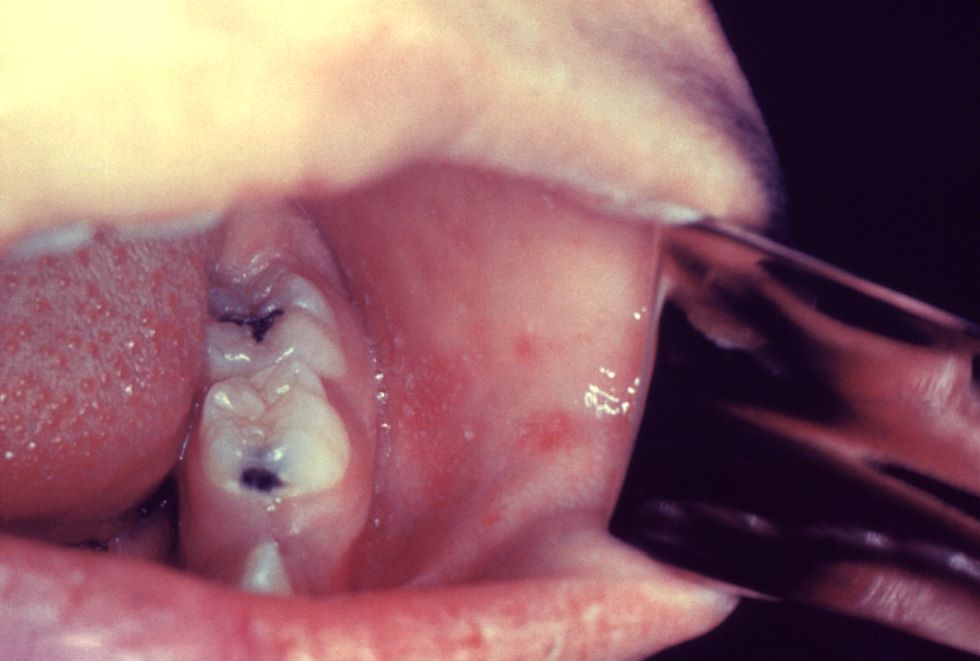
- Prodrome of upper respiratory tract symptoms which starts 4-21 days after exposure (usually 7-10 days) and lasts for 1-2 weeks.
- Paroxysmal cough with the following features:
- Is worse at night and may be associated with a short expiratory burst followed by an inspiratory gasp (the ‘whoop’).
- Is violent and uncontrolled, due to attempts to bring up thick mucus. This is not always successful, so the cough may appear dry as mucus remains in the bronchial tree.
- May be triggered by cold or noise.
- Associated with post-cough vomiting, with a risk of cyanosis in children and syncope in adults.
- Cough gets worse for 1-2 weeks, stays at the same frequency for another 2-3 weeks and then gradually gets less often. Although pertussis is colloquially known as the ‘100 day cough’, the paroxysmal phase of the cough usually lasts for no longer than 10 weeks, more commonly for six weeks. There is then a convalescent phase lasting 2-3 weeks, in which the cough gradually improves. If another respiratory tract infection is contracted in the few months after pertussis, paroxysm of coughing may recur.
- There is usually no fever.
Both measles and pertussis are notifiable at the point of
suspicion – public health can be notified online, and they will then get in
touch with the patient to arrange a diagnostic test if it is thought to be
appropriate. Treatment for measles is supportive, with admission if a
complication is suspected. Pertussis can be treated with a macrolide, but this
is only effective if started within 21 days of onset of the cough. If
macrolides are contraindicated, or not tolerated, the second-line antibiotic is
co-trimoxazole. This cannot be used in pregnant women; a discussion with
microbiology would be wise if treating a pregnant woman with pertussis and a
macrolide allergy. If a patient with suspected measles or pertussis does need
admission then it needs to be made clear to the admitting team what your
suspicion is, so that they can arrange appropriate isolation. Both infections
are extremely contagious, with each person who has measles infecting 15 - 20
others3, and each person with pertussis infecting 12-17 others.10
References [all accessed 17 June 2024]
1) UKHSA. Confirmed cases of pertussis in England by month. May 2024.
2) UKHSA. Latest measles statistics published. May 2024.
3) NICE CKS. Measles. Jan 2024.
4) NICE CKS. Whooping cough. March 2024.
5) UKHSA. Pertussis: the green book, chapter 24. April 2016.
6) UKHSA. Measles: the green book, chapter 21. Dec 2019.
7) UKHSA. Confirmed cases of measles in England by month, age and region: 2023. May 2024.
8) National Foundation for Infectious Diseases. Measles. May 2024.
9) UKHSA. What is whooping cough and is there a vaccine? April 2024.
10) Harrington L, Aris E, Bhavsar A. Burden of Pertussis in Adults Aged 50 Years and Older: A Retrospective Database Study in England. Infect Dis Ther. 2023 Apr;12(4):1103-1118.
11) NHSE. NHS urges young adults to catch up on missed MMR vaccine. March 2024.
12) NHS Lothian. Over 1,300 children and young people catch-up on their MMR vaccines in Lothian. March 2024.
13) Welsh Government. Call for all parents in Wales to urgently check their children’s MMR vaccination status amid rising concerns over measles. Feb 2024.
14) HSC Public Health Agency. MMR vaccine catch-up to tackle threat of measles. Feb 2024.
Written by Dr Emma Nash
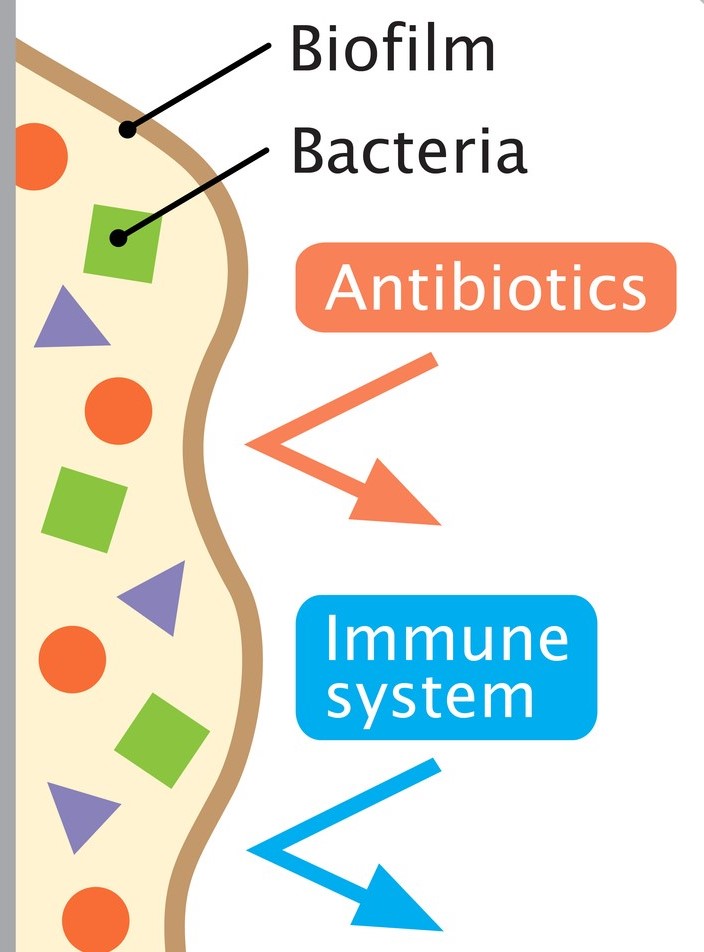
Cough is one of the most frequent reasons for presentation to primary care, particularly in children. Acute cough is most commonly caused by upper respiratory tract infections; chronic cough (over four weeks) is typically attributed to recurrent respiratory tract infection, asthma or pertussis. However, a wet cough lasting more than four weeks should prompt consideration of protracted bacterial bronchitis, also known as persistent bacterial bronchitis (PBB). First described in a study in 2006, the condition is being increasingly recognised clinically. However, there is no clarity on the underlying mechanisms, making definitive diagnostic and therapeutic guidance elusive. Proposed pathophysiological contributory factors include impaired mucociliary clearance, systemic immune function defects (raised NK-cell levels, altered expression of neutrophil-related mediators), and airway anomalies (tracheobronchomalacia). It is also thought that bacteria may produce a biofilm in the airways. This is a secreted matrix that enhances bacterial attachment to cells and facilitates access to nutrients. It also decreases antibiotic penetration, thus protecting the bacteria and making them harder to eradicate with standard length courses of treatment.
PBB is initially difficult to distinguish from a viral upper respiratory tract infection. The cough is productive, but the child is systemically well. Although wheeze is variably reported, the evidence is that there is a limited response to bronchodilator therapy, if any. Chest examination is typically normal, but if a wheeze is heard, it is monophonic rather than the polyphonic wheeze found in asthma. Sinus and ear disease is absent.
PBB is a clinical diagnosis. It may be suspected when the first two of the European Respiratory Society criteria are met, and confirmed when all three are met:
1. Presence of continuous chronic wet or productive cough (>four weeks’ duration).
2. Absence of symptoms or signs suggestive of other causes of wet or productive cough (see box 1).
3. Cough resolved following a two-to-four-week course of an appropriate oral antibiotic.
|
Box 1:
Pointers suggestive of causes other than PBB.
|
The median age of development is 10 months – 4.8 years and it affects more males than females. PBB is no more common in children with an atopic history, but one study has shown that children who attend childcare are significantly more likely to develop PBB than those who do not. Children with an airway malacia are at increased risk of developing the condition as it is harder to clear mucus when the airways tend to collapse. If this is an underlying factor for a particular child, giving a bronchodilator may exacerbate the symptoms as the airways relax even further. PBB is frequently misdiagnosed, or inadequately treated, resulting in a persistence of symptoms and potential structural damage in the form of bronchiectasis.
Most children who have PBB are not able to expectorate sputum for culture due to their age, so treatment is based on the sensitivity profiles of the typical pathogens. Haemophilus influenzae is the most common causative organism, followed by Streptococcus pneumoniae and Moraxella catarrhalis. However, sputum culture should be sent, if feasible.
 Previously, antibiotic treatment courses have been longer, and there has been much debate over optimal drug choice and duration. More recent evidence has shown that two weeks may be adequate in many cases. A further two weeks should be prescribed if the child has improved significantly but is not completely cough free. A minority will need longer courses, but if this is the case, there should be a high index of suspicion for more severe disease such as chronic suppurative lung disease (e.g. cystic fibrosis) or bronchiectasis. Therefore, failure of the cough to respond to four weeks of antibiotics should prompt discussion with a paediatrician for consideration of next steps and whether investigation is needed for underlying causes. A reasonable pathway in primary care would therefore be that a GP suspects PBB because the first two criteria are met, treats with two weeks of antibiotics (and a further two if there is no improvement) and then seek specialist advice if the cough persists after four weeks of antibiotics. Chest x-ray is generally only needed if there is a specific reason, such as focal chest signs of concern.
Previously, antibiotic treatment courses have been longer, and there has been much debate over optimal drug choice and duration. More recent evidence has shown that two weeks may be adequate in many cases. A further two weeks should be prescribed if the child has improved significantly but is not completely cough free. A minority will need longer courses, but if this is the case, there should be a high index of suspicion for more severe disease such as chronic suppurative lung disease (e.g. cystic fibrosis) or bronchiectasis. Therefore, failure of the cough to respond to four weeks of antibiotics should prompt discussion with a paediatrician for consideration of next steps and whether investigation is needed for underlying causes. A reasonable pathway in primary care would therefore be that a GP suspects PBB because the first two criteria are met, treats with two weeks of antibiotics (and a further two if there is no improvement) and then seek specialist advice if the cough persists after four weeks of antibiotics. Chest x-ray is generally only needed if there is a specific reason, such as focal chest signs of concern.
Unfortunately, no nationally accepted guidelines exist on treatment choice. The most commonly used antibiotic is oral co-amoxiclav but alternatives such as oral second or third generation cephalosporins, trimethoprim-sulfamethoxazole or a macrolide may be used when there is a history of an IgE-mediated reaction to penicillin.
It is worth noting that recurrent episodes are common, occurring in up to 76% of cases. Antibiotic prophylaxis may be helpful in children who have more than three recurrences in twelve months. Common choices for prophylaxis are azithromycin three days a week, or co-trimoxazole daily, but this would be on specialist advice.
References
American Thoracic Society. Protracted Bacterial Bronchitis (PBB) in Children. Am J Respir Crit Care Med. 2018; 198, P11-P12. View patient education sheet on Protracted Bacterial Bronchitis (PBB) in Children. [accessed 20 March 2024]
Bergmann M, Haasenritter J, Beidatsch D, et al. Coughing children in family practice and primary care: a systematic review of prevalence, aetiology and prognosis. BMC Pediatr. 2021 Jun 4;21(1):260.
Di Filippo P, Scaparrotta A, Petrosino Mi et al. An underestimated cause of chronic cough: The Protracted Bacterial Bronchitis. Ann Thorac Med. 2018 Jan-Mar;13(1):7-13.
Kansra S. Diagnosis and Management of Children with Protracted Bacterial Bronchitis PBB. Sheffield Children’s Hospital NHS Trust. 2017. Download guideline on Diagnosis and Management of Children with Protracted Bacterial Bronchitis PBB. [accessed 20 March 2024]
Kantar A, Chang A, Shields MD, et al. ERS statement on protracted bacterial bronchitis in children. European Respiratory Journal 2017 Aug; 50(2):1602139.
Dr Dirk Pilat
Rabies (from latin ‘to rage’) is a zoonotic, mostly fatal disease caused by a neurotropic virus of the lyssavirus genus.
The disease was first described in Egypt around 2300 BCE and is currently endemic in over 150 countries, with the highest risk of exposure in Africa and Asia. Dogs are the principal host in Africa, Asia and America, but the virus can be present in other wild animals, such as bats, cats, skunks or racoons. The most common way of transmission is the bite of an infected dog or cat, though handling of infected carcasses can cause infection too.
Figure 1. 3D still
showing rabies virus by www.scientificanimations.com/is
licenced under CC
BY-SA 4.0 DEED
Worldwide, around 59,000 people die of rabies each year, mainly in Africa and Asia. In the UK there have been reportedly 26 fatalities since 1946, with the most recent in 2018. There is recent qualitative and quantitative evidence that the perceived risk of exposure to rabies for travellers is low, with vaccination often avoided due to cost implications.
Before the SARS-CoV-2 pandemic, circa 2000 people required post-exposure treatment in England per year, of which 12% were potentially exposed to the two bat species in the UK who harbour the virus. The disease remains prevalent in some countries in Eastern Europe.
After an incubation time of typically three to 12 weeks, infection with the virus can cause a slow but progressive encephalitis with early symptoms being headache, fever, and malaise. The patient may then develop the characteristic symptoms of hydrophobia, hallucinations, and features of mania before progressing to paralysis and coma. Rabies is almost always fatal, and there is currently no specific treatment other than symptom control.
Pre-exposure prophylaxis via vaccination before travelling (or for those with occupational hazards) into endemic areas is a safe and effective method of protecting people from rabies, with the Green Book recommending an individual risk assessment of potential exposure prior to travelling.
Post-exposure management should consist of wound treatment (running tap water over the wound for several minutes, washing with soap or detergent and application of a dressing with disinfectants (such as 40 to 70% alcohol, tincture, or aqueous solution of povidone-iodine) and treatment with either an accelerated course of rabies vaccine or administration of human rabies immunoglobulin. The decision about which course of post-exposure treatment to administer depends on the categories of exposure, which animal was involved and in which country the incident occurred. Advice on who to contact in a suspected case of rabies can be obtained in this table from the Green Book.
Guidance Rabies: the green book, chapter 27, p11. Contains public sector information licensed under the Open Government Licence v3.0.
 Tetanus is a disease caused by the neurotoxin produced by the bacterium Clostridium tetani. The spores of the bacterium are ubiquitous in soil throughout the world and enter contaminated wounds or minor abrasions. In low- and middle-income countries, both maternal (tetanus during pregnancy or within 6 weeks of the end of pregnancy via birth, miscarriage, or abortion) and neonatal tetanus (infection occurs via the umbilical stump) are likely significantly under-reported, but it is estimated that in 2015 about 35,000 deaths were due to neonatal tetanus and 57,000 adults were reported to have died. In high income countries, older people and people who inject drugs are the most likely to contract the disease. Migrants from countries with poor vaccination programmes can be particularly susceptible. Between 2001 and 2022, 147 cases of tetanus were reported to the UK Health Security Agency.
Tetanus is a disease caused by the neurotoxin produced by the bacterium Clostridium tetani. The spores of the bacterium are ubiquitous in soil throughout the world and enter contaminated wounds or minor abrasions. In low- and middle-income countries, both maternal (tetanus during pregnancy or within 6 weeks of the end of pregnancy via birth, miscarriage, or abortion) and neonatal tetanus (infection occurs via the umbilical stump) are likely significantly under-reported, but it is estimated that in 2015 about 35,000 deaths were due to neonatal tetanus and 57,000 adults were reported to have died. In high income countries, older people and people who inject drugs are the most likely to contract the disease. Migrants from countries with poor vaccination programmes can be particularly susceptible. Between 2001 and 2022, 147 cases of tetanus were reported to the UK Health Security Agency.Figure 2.Opisthotonus in a patient suffering from tetanus - Painting by Sir Charles Bell - 1809. In public domain.
Under anaerobic conditions the spores germinate, and the newly formed bacteria produce a highly toxic neurotoxin that is transported retrogradely along the axonal pathways, reducing motor nerve inhibition. After an incubation time of up to 21 days, localised or general spasms (such as back arching, known as opisthotonos) can occur, with trismus and ‘risus sardonicus’ being early signs. This can be associated with dysphagia due to laryngeal and pharyngeal spasms. Testing is available via wound samples or serology but should not delay treatment. The UK Health Security Agency defines a probable case as “In the absence of a more likely diagnosis, an acute illness with muscle spasms or hypertonia, and diagnosis of tetanus by a health care provider”.
Treatment requires thorough cleaning and debridement of the wound, antibiotics and in severe cases mechanical ventilation under neuromuscular blocking agents.
Fortunately, a safe and efficient vaccine exists and has been in regular use since 1961. By the 1970s the disease had almost disappeared in children under 15. These days, it is part of the United Kingdom’s routine immunisation schedule.
 Patients with tetanus prone wounds might, depending on their
vaccination status, receive a reinforcing dose of tetanus vaccine and – if
indicated – a dose of human tetanus immunoglobulin after their wound has been
thoroughly cleaned. The Green Book categorises tetanus prone wounds as:
Patients with tetanus prone wounds might, depending on their
vaccination status, receive a reinforcing dose of tetanus vaccine and – if
indicated – a dose of human tetanus immunoglobulin after their wound has been
thoroughly cleaned. The Green Book categorises tetanus prone wounds as:
- puncture-type injuries acquired in a contaminated environment and likely therefore to contain tetanus spores e.g. gardening injuries and wounds containing foreign bodies
- compound fractures
- wounds or burns with systemic sepsis
- certain animal bites and scratches - although smaller bites from domestic pets are generally puncture injuries, animal saliva should not contain tetanus spores unless the animal has been routing in soil or lives in an agricultural setting.
High-risk tetanus wounds include any of the above with either:
- heavy contamination with material likely to contain tetanus spores e.g. soil, manure
- wounds or burns that show extensive devitalised tissue
- wounds or burns that require surgical intervention that is delayed for more than six hours are high risk even if the contamination was not initially heavy.
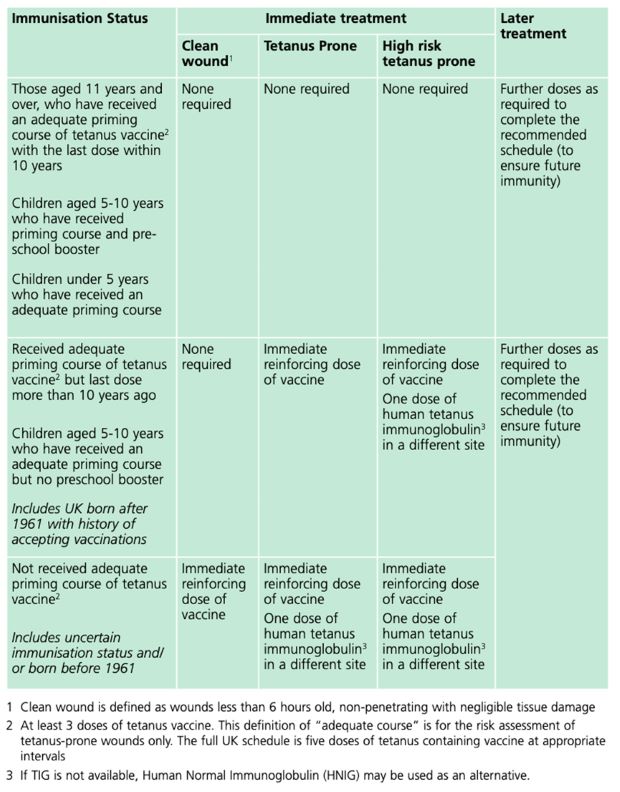
Guidance Tetanus: the green book, chapter 30, Table 30.1, p.10. Contains public sector information licensed under the Open Government Licence v3.0.
References
Marano, C et al. (2019). Perceptions of rabies risk: a survey of travellers and travel clinics from Canada, Germany, Sweden and the UK. Journal of Travel Medicine, Vol 26, Suppl 1, S3–S9, DOI: 10.1093/jtm/tay062
Singh,R et al.(2017). Rabies – epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review, Veterinary Quarterly, 37:1, 212-251, DOI: 10.1080/01652176.2017.1343516
United Kingdom Health Security Agency (2023). Guidance on the management of suspected tetanus cases and the assessment and management of tetanus-prone wounds. https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals/guidance-on-the-management-of-suspected-tetanus-cases-and-the-assessment-and-management-of-tetanus-prone-wounds (Accessed 14.11.2023)
United Kingdom Health Security Agency (2023). Rabies: the green book, chapter 27. https://www.gov.uk/government/publications/rabies-the-green-book-chapter-27 (Accessed 13.11.2023)
United Kingdom Health Security Agency (2022). Tetanus: the green book, chapter 30. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1080599/Green_Book_on_immunisation_chapter_30_tetanus.pdf (Accessed 14.11.2023)
Yen, LM; Thwaites, CL (2019). Tetanus. Lancet 2019; 393: 1657–68 http://dx.doi.org/10.1016/S0140-6736(18)33131-3