_ RCGP Learning
User blog: _ RCGP Learning
Written by Dr Emma Nash
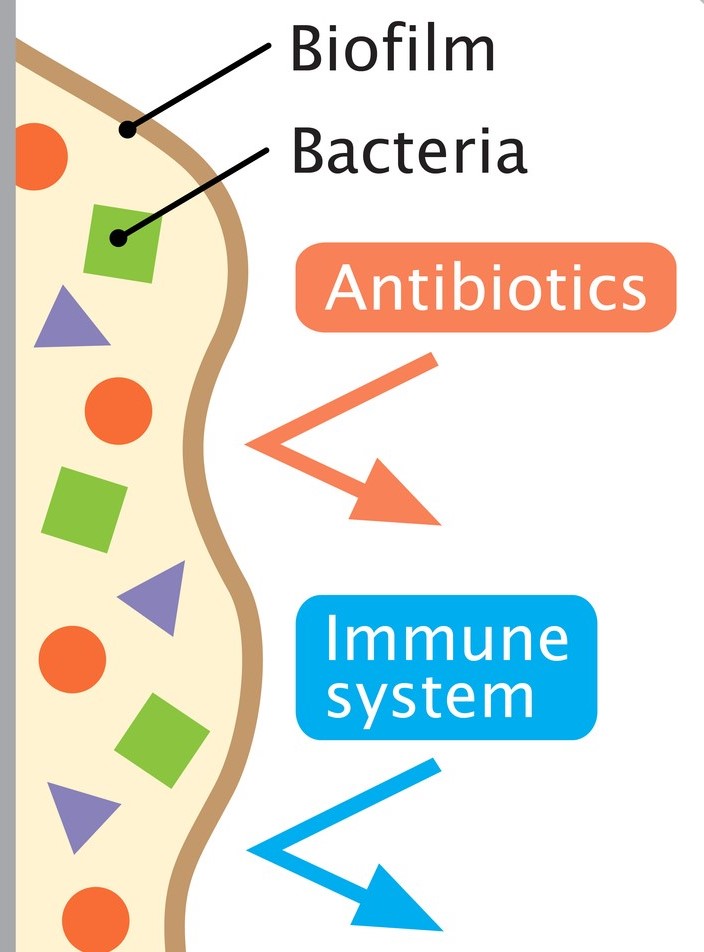
Cough is one of the most frequent reasons for presentation to primary care, particularly in children. Acute cough is most commonly caused by upper respiratory tract infections; chronic cough (over four weeks) is typically attributed to recurrent respiratory tract infection, asthma or pertussis. However, a wet cough lasting more than four weeks should prompt consideration of protracted bacterial bronchitis, also known as persistent bacterial bronchitis (PBB). First described in a study in 2006, the condition is being increasingly recognised clinically. However, there is no clarity on the underlying mechanisms, making definitive diagnostic and therapeutic guidance elusive. Proposed pathophysiological contributory factors include impaired mucociliary clearance, systemic immune function defects (raised NK-cell levels, altered expression of neutrophil-related mediators), and airway anomalies (tracheobronchomalacia). It is also thought that bacteria may produce a biofilm in the airways. This is a secreted matrix that enhances bacterial attachment to cells and facilitates access to nutrients. It also decreases antibiotic penetration, thus protecting the bacteria and making them harder to eradicate with standard length courses of treatment.
PBB is initially difficult to distinguish from a viral upper respiratory tract infection. The cough is productive, but the child is systemically well. Although wheeze is variably reported, the evidence is that there is a limited response to bronchodilator therapy, if any. Chest examination is typically normal, but if a wheeze is heard, it is monophonic rather than the polyphonic wheeze found in asthma. Sinus and ear disease is absent.
PBB is a clinical diagnosis. It may be suspected when the first two of the European Respiratory Society criteria are met, and confirmed when all three are met:
1. Presence of continuous chronic wet or productive cough (>four weeks’ duration).
2. Absence of symptoms or signs suggestive of other causes of wet or productive cough (see box 1).
3. Cough resolved following a two-to-four-week course of an appropriate oral antibiotic.
|
Box 1:
Pointers suggestive of causes other than PBB.
|
The median age of development is 10 months – 4.8 years and it affects more males than females. PBB is no more common in children with an atopic history, but one study has shown that children who attend childcare are significantly more likely to develop PBB than those who do not. Children with an airway malacia are at increased risk of developing the condition as it is harder to clear mucus when the airways tend to collapse. If this is an underlying factor for a particular child, giving a bronchodilator may exacerbate the symptoms as the airways relax even further. PBB is frequently misdiagnosed, or inadequately treated, resulting in a persistence of symptoms and potential structural damage in the form of bronchiectasis.
Most children who have PBB are not able to expectorate sputum for culture due to their age, so treatment is based on the sensitivity profiles of the typical pathogens. Haemophilus influenzae is the most common causative organism, followed by Streptococcus pneumoniae and Moraxella catarrhalis. However, sputum culture should be sent, if feasible.
 Previously, antibiotic treatment courses have been longer, and there has been much debate over optimal drug choice and duration. More recent evidence has shown that two weeks may be adequate in many cases. A further two weeks should be prescribed if the child has improved significantly but is not completely cough free. A minority will need longer courses, but if this is the case, there should be a high index of suspicion for more severe disease such as chronic suppurative lung disease (e.g. cystic fibrosis) or bronchiectasis. Therefore, failure of the cough to respond to four weeks of antibiotics should prompt discussion with a paediatrician for consideration of next steps and whether investigation is needed for underlying causes. A reasonable pathway in primary care would therefore be that a GP suspects PBB because the first two criteria are met, treats with two weeks of antibiotics (and a further two if there is no improvement) and then seek specialist advice if the cough persists after four weeks of antibiotics. Chest x-ray is generally only needed if there is a specific reason, such as focal chest signs of concern.
Previously, antibiotic treatment courses have been longer, and there has been much debate over optimal drug choice and duration. More recent evidence has shown that two weeks may be adequate in many cases. A further two weeks should be prescribed if the child has improved significantly but is not completely cough free. A minority will need longer courses, but if this is the case, there should be a high index of suspicion for more severe disease such as chronic suppurative lung disease (e.g. cystic fibrosis) or bronchiectasis. Therefore, failure of the cough to respond to four weeks of antibiotics should prompt discussion with a paediatrician for consideration of next steps and whether investigation is needed for underlying causes. A reasonable pathway in primary care would therefore be that a GP suspects PBB because the first two criteria are met, treats with two weeks of antibiotics (and a further two if there is no improvement) and then seek specialist advice if the cough persists after four weeks of antibiotics. Chest x-ray is generally only needed if there is a specific reason, such as focal chest signs of concern.
Unfortunately, no nationally accepted guidelines exist on treatment choice. The most commonly used antibiotic is oral co-amoxiclav but alternatives such as oral second or third generation cephalosporins, trimethoprim-sulfamethoxazole or a macrolide may be used when there is a history of an IgE-mediated reaction to penicillin.
It is worth noting that recurrent episodes are common, occurring in up to 76% of cases. Antibiotic prophylaxis may be helpful in children who have more than three recurrences in twelve months. Common choices for prophylaxis are azithromycin three days a week, or co-trimoxazole daily, but this would be on specialist advice.
References
American Thoracic Society. Protracted Bacterial Bronchitis (PBB) in Children. Am J Respir Crit Care Med. 2018; 198, P11-P12. View patient education sheet on Protracted Bacterial Bronchitis (PBB) in Children. [accessed 20 March 2024]
Bergmann M, Haasenritter J, Beidatsch D, et al. Coughing children in family practice and primary care: a systematic review of prevalence, aetiology and prognosis. BMC Pediatr. 2021 Jun 4;21(1):260.
Di Filippo P, Scaparrotta A, Petrosino Mi et al. An underestimated cause of chronic cough: The Protracted Bacterial Bronchitis. Ann Thorac Med. 2018 Jan-Mar;13(1):7-13.
Kansra S. Diagnosis and Management of Children with Protracted Bacterial Bronchitis PBB. Sheffield Children’s Hospital NHS Trust. 2017. Download guideline on Diagnosis and Management of Children with Protracted Bacterial Bronchitis PBB. [accessed 20 March 2024]
Kantar A, Chang A, Shields MD, et al. ERS statement on protracted bacterial bronchitis in children. European Respiratory Journal 2017 Aug; 50(2):1602139.
Dr Dirk Pilat
Rabies (from latin ‘to rage’) is a zoonotic, mostly fatal disease caused by a neurotropic virus of the lyssavirus genus.
The disease was first described in Egypt around 2300 BCE and is currently endemic in over 150 countries, with the highest risk of exposure in Africa and Asia. Dogs are the principal host in Africa, Asia and America, but the virus can be present in other wild animals, such as bats, cats, skunks or racoons. The most common way of transmission is the bite of an infected dog or cat, though handling of infected carcasses can cause infection too.
Figure 1. 3D still
showing rabies virus by www.scientificanimations.com/is
licenced under CC
BY-SA 4.0 DEED
Worldwide, around 59,000 people die of rabies each year, mainly in Africa and Asia. In the UK there have been reportedly 26 fatalities since 1946, with the most recent in 2018. There is recent qualitative and quantitative evidence that the perceived risk of exposure to rabies for travellers is low, with vaccination often avoided due to cost implications.
Before the SARS-CoV-2 pandemic, circa 2000 people required post-exposure treatment in England per year, of which 12% were potentially exposed to the two bat species in the UK who harbour the virus. The disease remains prevalent in some countries in Eastern Europe.
After an incubation time of typically three to 12 weeks, infection with the virus can cause a slow but progressive encephalitis with early symptoms being headache, fever, and malaise. The patient may then develop the characteristic symptoms of hydrophobia, hallucinations, and features of mania before progressing to paralysis and coma. Rabies is almost always fatal, and there is currently no specific treatment other than symptom control.
Pre-exposure prophylaxis via vaccination before travelling (or for those with occupational hazards) into endemic areas is a safe and effective method of protecting people from rabies, with the Green Book recommending an individual risk assessment of potential exposure prior to travelling.
Post-exposure management should consist of wound treatment (running tap water over the wound for several minutes, washing with soap or detergent and application of a dressing with disinfectants (such as 40 to 70% alcohol, tincture, or aqueous solution of povidone-iodine) and treatment with either an accelerated course of rabies vaccine or administration of human rabies immunoglobulin. The decision about which course of post-exposure treatment to administer depends on the categories of exposure, which animal was involved and in which country the incident occurred. Advice on who to contact in a suspected case of rabies can be obtained in this table from the Green Book.
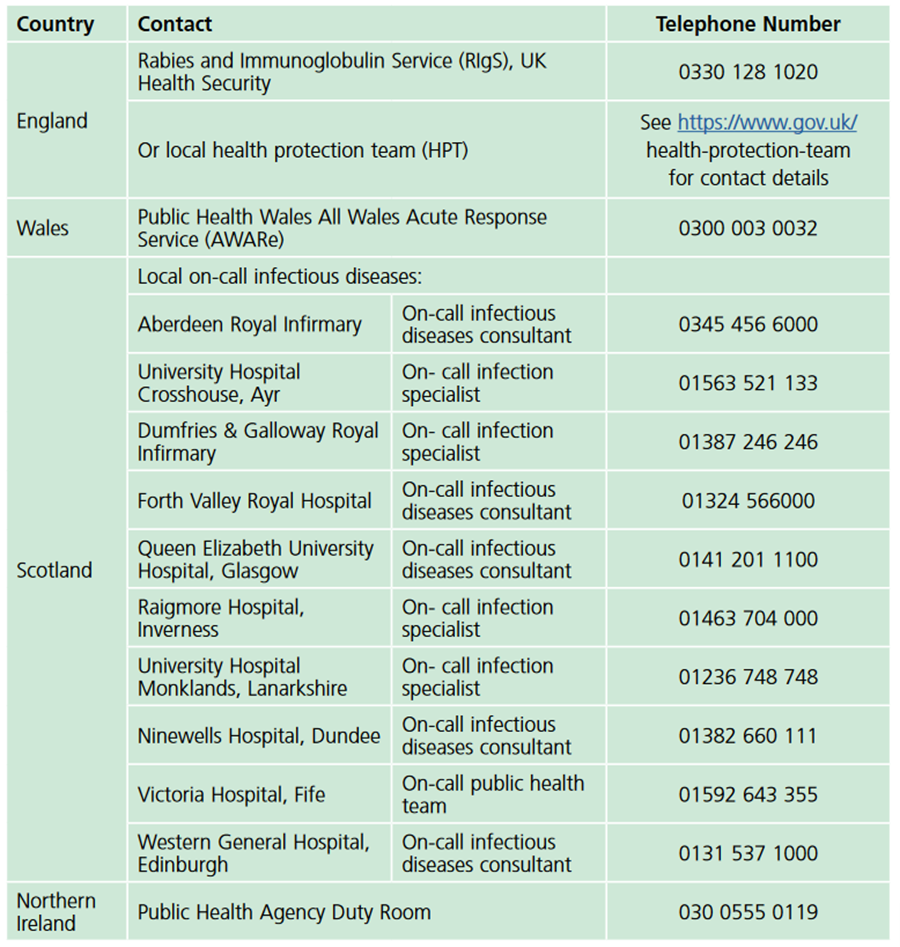
Guidance Rabies: the green book, chapter 27, p11. Contains public sector information licensed under the Open Government Licence v3.0.
 Tetanus is a disease caused by the neurotoxin produced by the bacterium Clostridium tetani. The spores of the bacterium are ubiquitous in soil throughout the world and enter contaminated wounds or minor abrasions. In low- and middle-income countries, both maternal (tetanus during pregnancy or within 6 weeks of the end of pregnancy via birth, miscarriage, or abortion) and neonatal tetanus (infection occurs via the umbilical stump) are likely significantly under-reported, but it is estimated that in 2015 about 35,000 deaths were due to neonatal tetanus and 57,000 adults were reported to have died. In high income countries, older people and people who inject drugs are the most likely to contract the disease. Migrants from countries with poor vaccination programmes can be particularly susceptible. Between 2001 and 2022, 147 cases of tetanus were reported to the UK Health Security Agency.
Tetanus is a disease caused by the neurotoxin produced by the bacterium Clostridium tetani. The spores of the bacterium are ubiquitous in soil throughout the world and enter contaminated wounds or minor abrasions. In low- and middle-income countries, both maternal (tetanus during pregnancy or within 6 weeks of the end of pregnancy via birth, miscarriage, or abortion) and neonatal tetanus (infection occurs via the umbilical stump) are likely significantly under-reported, but it is estimated that in 2015 about 35,000 deaths were due to neonatal tetanus and 57,000 adults were reported to have died. In high income countries, older people and people who inject drugs are the most likely to contract the disease. Migrants from countries with poor vaccination programmes can be particularly susceptible. Between 2001 and 2022, 147 cases of tetanus were reported to the UK Health Security Agency.Figure 2.Opisthotonus in a patient suffering from tetanus - Painting by Sir Charles Bell - 1809. In public domain.
Under anaerobic conditions the spores germinate, and the newly formed bacteria produce a highly toxic neurotoxin that is transported retrogradely along the axonal pathways, reducing motor nerve inhibition. After an incubation time of up to 21 days, localised or general spasms (such as back arching, known as opisthotonos) can occur, with trismus and ‘risus sardonicus’ being early signs. This can be associated with dysphagia due to laryngeal and pharyngeal spasms. Testing is available via wound samples or serology but should not delay treatment. The UK Health Security Agency defines a probable case as “In the absence of a more likely diagnosis, an acute illness with muscle spasms or hypertonia, and diagnosis of tetanus by a health care provider”.
Treatment requires thorough cleaning and debridement of the wound, antibiotics and in severe cases mechanical ventilation under neuromuscular blocking agents.
Fortunately, a safe and efficient vaccine exists and has been in regular use since 1961. By the 1970s the disease had almost disappeared in children under 15. These days, it is part of the United Kingdom’s routine immunisation schedule.
 Patients with tetanus prone wounds might, depending on their
vaccination status, receive a reinforcing dose of tetanus vaccine and – if
indicated – a dose of human tetanus immunoglobulin after their wound has been
thoroughly cleaned. The Green Book categorises tetanus prone wounds as:
Patients with tetanus prone wounds might, depending on their
vaccination status, receive a reinforcing dose of tetanus vaccine and – if
indicated – a dose of human tetanus immunoglobulin after their wound has been
thoroughly cleaned. The Green Book categorises tetanus prone wounds as:
- puncture-type injuries acquired in a contaminated environment and likely therefore to contain tetanus spores e.g. gardening injuries and wounds containing foreign bodies
- compound fractures
- wounds or burns with systemic sepsis
- certain animal bites and scratches - although smaller bites from domestic pets are generally puncture injuries, animal saliva should not contain tetanus spores unless the animal has been routing in soil or lives in an agricultural setting.
High-risk tetanus wounds include any of the above with either:
- heavy contamination with material likely to contain tetanus spores e.g. soil, manure
- wounds or burns that show extensive devitalised tissue
- wounds or burns that require surgical intervention that is delayed for more than six hours are high risk even if the contamination was not initially heavy.
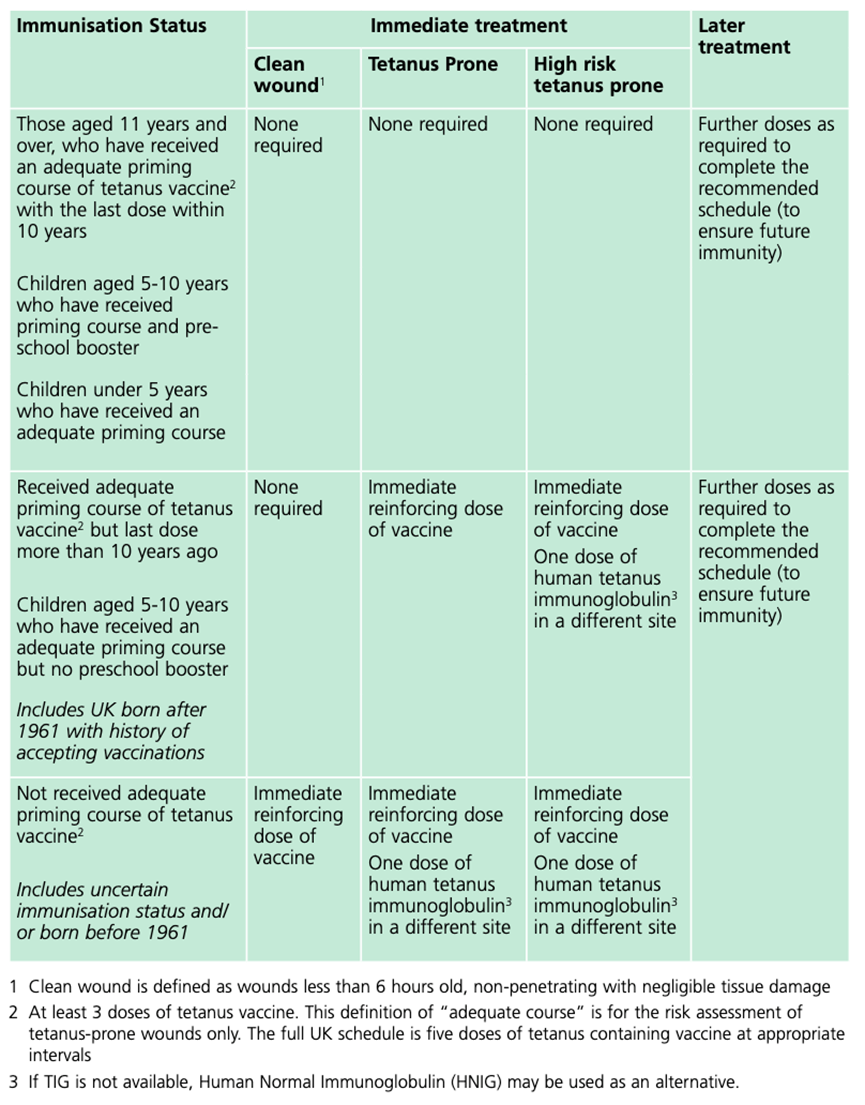
Guidance Tetanus: the green book, chapter 30, Table 30.1, p.10. Contains public sector information licensed under the Open Government Licence v3.0.
References
Marano, C et al. (2019). Perceptions of rabies risk: a survey of travellers and travel clinics from Canada, Germany, Sweden and the UK. Journal of Travel Medicine, Vol 26, Suppl 1, S3–S9, DOI: 10.1093/jtm/tay062
Singh,R et al.(2017). Rabies – epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive review, Veterinary Quarterly, 37:1, 212-251, DOI: 10.1080/01652176.2017.1343516
United Kingdom Health Security Agency (2023). Guidance on the management of suspected tetanus cases and the assessment and management of tetanus-prone wounds. https://www.gov.uk/government/publications/tetanus-advice-for-health-professionals/guidance-on-the-management-of-suspected-tetanus-cases-and-the-assessment-and-management-of-tetanus-prone-wounds (Accessed 14.11.2023)
United Kingdom Health Security Agency (2023). Rabies: the green book, chapter 27. https://www.gov.uk/government/publications/rabies-the-green-book-chapter-27 (Accessed 13.11.2023)
United Kingdom Health Security Agency (2022). Tetanus: the green book, chapter 30. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1080599/Green_Book_on_immunisation_chapter_30_tetanus.pdf (Accessed 14.11.2023)
Yen, LM; Thwaites, CL (2019). Tetanus. Lancet 2019; 393: 1657–68 http://dx.doi.org/10.1016/S0140-6736(18)33131-3
Written by Dr Dirk Pilat
The Hepatitis E virus (HEV) is the most common cause for
acute viral hepatitis worldwide, a fact that is not widely known in the United
Kingdom. It is a small RNA virus that has various genotypes, though the most
important are HEV 1&2 – the most common types in low-income countries – and
HEV 3, which occurs in Europe, the Americas and Australia.
HEV is responsible for about 20 million infections worldwide each year, of which 3 million will be symptomatic and 56,000 lethal. In the United Kingdom, the UK Health Security Agency (UKHSA) follows up confirmed Hepatitis E infections to investigate non-travel related cases of HEV in England and Wales while Public Health Scotland (PHS) monitors HEV infections within their territory.
HEV 1&2 are obligate human pathogens and usually transmitted faecal – orally by contaminated drinking water, while HEV 3 is usually transmitted via inadequately cooked pork, molluscs, or seafood. 90% of pigs in the UK have antibodies to HEV, and around 20% have been known to have an active infection at the time of slaughter. Within Europe the prevalence varies, though there are geographic hotspots with human seroprevalence of >50%. In 2019, UKHSA reported 1202 confirmed HEV infections, PHS reported 158 (>100 cases more than Hepatis A) though due to its often asymptomatic nature, it is reported to be significantly underdiagnosed.
The presentation of acute HEV infections depends on the location of the patient and the genome responsible: In resource poor countries it usually affects young people 15-35 years of age who experience an acute, self-limiting hepatitis due to HEV1 (Asia) or 2 (Central America). Preterm women are particularly affected, with papers quoting mortality rates of up to 25% and preterm deliveries in 66%.
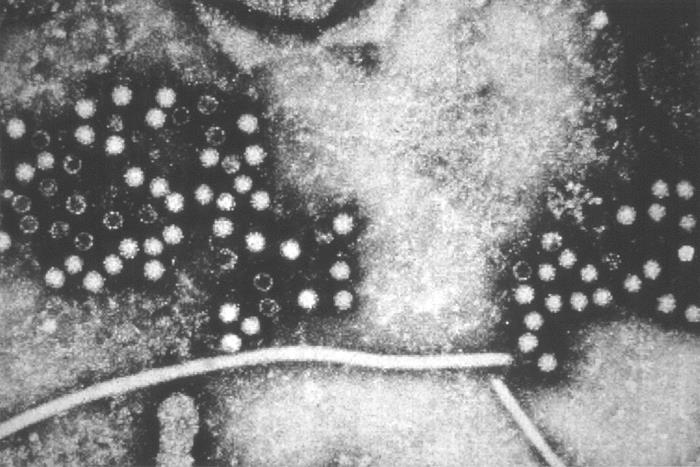
This transmission electron micrograph depicts numerous, spheroid-shaped, hepatitis-E viruses (HEV), also known as Orthohepevirus A. Image used with permission from Centers for Disease Control and Prevention (CDC).
• ALT > 300 IU/L
• Clinical suspicion of drug
induced liver injury
• Decompensated chronic liver
disease (regardless of LFT results)
• Guillain–Barré syndrome
(regardless of LFT results)
• Neuralgic amyotrophy (regardless
of LFT results)
• Patients with unexplained acute
neurology and a raised ALT
During the first 2 weeks of hepatitis E illness:
- avoid preparing food for others
- limit contact with others if possible, especially pregnant women, or people with chronic liver disease
- wash hands thoroughly with soap and warm water and then dry properly after contact with an infected person
- wash hands after going to the toilet, before preparing, serving and eating food
Pregnant women should contact their obstetric team for advice.
As there is currently no licensed vaccine for Hepatitis E in the United Kingdom, prevention is key. UKHSA suggests:
- cooking meat and meat products thoroughly
- avoid eating raw or undercooked meat and shellfish
- washing hands thoroughly before preparing, serving and eating food.
When travelling to countries with poor sanitation:
- boil all drinking water, including water for brushing teeth
- avoid eating raw or undercooked meat and shellfish.
In primary care, a good travel history can be key to diagnose a patient with an unexplained rise in liver function tests and prodromal symptoms.
References:
Aslan, TA; Balaban, HY (2020). Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World Journal of Gastroenterology. October 7; 26(37): 5543-5560
Horvatits, T; Schulze zur Wisch, J et al. (2019). The clinical perspective on Hepatitis E. Viruses 11, 617
Public Health Scotland: Ten-year gastrointestinal and zoonoses data tables (2023). https://publichealthscotland.scot/publications/ten-year-gastrointestinal-and-zoonoses-data-tables/ten-year-gastrointestinal-and-zoonoses-data-tables-march-2023/ (Accessed 15.08.2023)
UKHSA: Hepatitis E: symptoms, transmission, treatment and prevention (2020). https://www.gov.uk/government/publications/hepatitis-e-symptoms-transmission-prevention-treatment/hepatitis-e-symptoms-transmission-treatment-and-prevention (Accessed 15.08.2023)
Webb, GW; Dalton, HR (2019). Hepatitis E: an underestimated emerging threat. Therapeutic Advances in Infectious Disease Vol. 6: 1–18
 Written by Dr Toni Hazell
Written by Dr Toni Hazell
Menopause is defined as a biological stage in a woman’s life when menstruation stops permanently, due to the loss of ovarian follicular activity. It is a retrospective diagnosis, made 12 months after the last period, which often follows several years of perimenopause in which periods can be irregular and menopausal symptoms may be felt. The average age of the menopause in the UK is 51. An early menopause is one that happens before 45. Premature ovarian insufficiency (POI) is defined as the transient or permanent loss of ovarian function below the age of 401. Care for women with POI should be holistic – as well as the risks to physical health, POI can be very distressing, particularly for those women who do not feel that they have completed their family.
A woman with POI may have an absolutely reduced number of ovarian follicles, or there may be remaining follicles which are not functioning properly. 90% of POI is idiopathic, but the following causes of a reduction or poor function of the follicles may be found in up to 10% of women2:
- Chromosomal disorders (e.g. Fragile X and Turner syndrome)
- Autoimmune disease (e.g. thyroiditis and Addison’s)
- Iatrogenic (e.g. due to the use of chemotherapy or pelvic radiotherapy or surgical after oophorectomy)
- Metabolic disorders (e.g. galactosaemia)
- Toxins such as cigarette smoke and some chemicals and pesticides can speed up follicle depletion
- Infection (e.g. mumps, TB and malaria – this is rare).
POI should be suspected in women who are under than 40 who have at least four months of amenorrhoea, with an estimated FSH level of more than 30 IU/L on two blood samples taken 4 – 6 weeks apart1,2,3. If the diagnosis is in doubt, then a referral should be done4. Referral might be to a specialist menopause clinic, to endocrinology or to gynaecology, depending on your specific concerns and what pathways you have available locally. A full history should be taken, including:
- a review of menopausal symptoms
- other possible causes (including pregnancy)
- lifestyle factors (smoking, alcohol, exercise, nutrition)
- the need for contraception (women with POI still have a small risk of ovulation)
- smear status
- family history of POI, venous thromboembolism or breast cancer
- the woman’s thoughts about HRT use
- any co-morbidities which might be relevant when considering HRT use
- osteoporosis risk – consider a baseline assessment of bone mineral density, with a repeat 2-3 years after diagnosis3.
Those who have worked in primary care for decades will be aware of the changing demand for hormone replacement therapy (HRT) over the years, with a significant decline in the early 2000s5, associated with concerns about cardiovascular risk and breast cancer, often based on studies looking at different populations from those who are prescribed HRT in the UK. This has been reversed in the last few years, with a 35% increase in HRT items prescribed from 2020/21 to 2021/226. It is vital that healthcare professionals understand the key difference between prescribing HRT for women who go through their menopause at a normal age, and those with POI, as the risk/benefit analysis is completely different. For women with POI, HRT is merely replacing the hormones that an average woman would have had naturally and reducing the excess risk of osteoporosis that comes with POI. For the vast majority there appears to be no excess risk of breast cancer arising from the use of HRT up to the age of 503. Both NICE4 and the British Menopause Society3 (BMS) are clear that this cohort of women should be offered HRT unless there is a clear absolute or relative contraindication, the main one being a history of a hormone sensitive cancer such as breast cancer. All the data that we have suggests that transdermal oestrogen does not increase the risk of venous thromboembolism (VTE) in women who have their menopause at a normal age3,4 – the BMS acknowledges the limited data in women with POI but says that the transdermal route should be considered in women with POI who are at an increased risk of VTE, for example due to obesity. Transdermal oestrogen, when used with micronised progesterone, is also ‘unlikely to significantly increase VTE risk above the individual’s intrinsic risk’7 for those who have a personal or family history of VTE.
The decision as to whether to refer a woman with POI to a specialist menopause clinic will depend on several factors. These may include the level of suspicion of an underlying cause, the confidence of the GP to manage POI, the woman’s desire for ongoing fertility, a need for specialist psychological input and any individual risk factors for HRT. For women with a history of a hormone sensitive cancer, a referral to discuss the risks and benefits of HRT would be sensible, and this discussion might usefully include her oncologist.
Women who go through their menopause at a normal age are advised to use contraception for one year after their last period (if that happens over the age of 50), or two years if their last period is under the age of 50. Women with POI have a higher risk of spontaneous ovulation and conception and have around a 5 – 10% change of spontaneous natural conception3. Contraception is therefore advised if they do not want to become pregnant and some will prefer to use combined hormonal contraception (CHC) instead of HRT – either are suitable options for oestrogen replacement (assuming no contraindications to combined hormonal contraception), although HRT may be more beneficial for bone health and cardiovascular risk3. They should continue to have smear tests at the normal frequency for their age. If a woman has no need for contraception (e.g. post sterilisation) and has no other licensed indications (e.g. menstrual symptoms) and is using CHC because she prefers it to HRT, then this will be unlicensed.
A diagnosis of POI can have physical, social, and
psychological impacts on a woman and her family so holistic care is important.
Signposting to a charity such as The Daisy Network8 and to reliable
sources of information such as the Women’s Health Concern9, Rock My
Menopause10, the RCOG menopause hub11, and articles on
the website Patient12 may help her to feel more in control. GPs who
wish to learn more about menopause might want to start with the RCGP course on
the subject, consisting of a half-hour eLearning module, a short screencast and
a podcast13. Access Menopause eLearning course via the following link https://elearning.rcgp.org.uk/course/info.php?id=663
Declaration of interests – Dr. Hazell does both paid and
unpaid work for the PCWHF, who host Rock My Menopause, and also works for the
website Patient.
References (all viewed 26.5.23)
1) NICE CKS. Menopause. Last updated September 2022. https://cks.nice.org.uk/topics/menopause/
2) Daisy network. What is POI. https://www.daisynetwork.org/about-poi/what-is-poi/
3) British Menopause Society. Premature Ovarian Insufficiency. April 2020. https://thebms.org.uk/publications/consensus-statements/premature-ovarian-insufficiency/
4) NICE. NG23. Menopause: diagnosis and management. Last updated December 2019. https://www.nice.org.uk/guidance/ng23
5) Cagnacci A, Venier M. The Controversial History of Hormone Replacement Therapy. Medicina (Kaunas). 2019 Sep 18;55(9):602
6) NHSBSA Statistics and Data Science. Hormone replacement therapy – England. October 2022. https://www.nhsbsa.nhs.uk/statistical-collections/hormone-replacement-therapy-england/hormone-replacement-therapy-england-april-2015-june-2022
7) Hamoda H, Panay N, Pedder H et al. The British Menopause Society & Women's Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod Health. 2020 Dec;26(4):181-209
8) The Daisy Network. https://www.daisynetwork.org/
9) Women’s Health Concern. https://www.womens-health-concern.org/
10) Rock My Menopause. Premature ovarian insufficiency factsheet. https://rockmymenopause.com/portfolio-item/premature-ovarian-insufficiency
11) RCOG. Menopause and later life. https://www.rcog.org.uk/for-the-public/menopause-and-later-life/
12) Patient. What it’s like to go through early menopause. Last updated June 2019. https://patient.info/news-and-features/what-its-like-to-go-through-early-menopause
13) RCGP eLearning. Menopause. September 2022. https://elearning.rcgp.org.uk/course/view.php?id=663
14) Eunice Kennedy Shriver National Institute of Child Health and Human Development. What causes POI? https://www.nichd.nih.gov/health/topics/poi/conditioninfo/causes
You might have read recently in the daily and medical press that there has been an outbreak of diphtheria among asylum seekers in England. Even though refugees are often young, physically fit males, they can have complex health needs after their long journeys, such as untreated communicable diseases. Since June 2022 there has been an increase in diphtheria cases, and as of December 2022 62 cases of diphtheria had been confirmed, with half of the cases being cutaneous. Before mass vaccination, diphtheria was once one of the most feared childhood diseases in the UK, with more than 61,000 cases and 3,283 deaths in 1940.
Figure 1 Corynebacteriæ diphtheriæ
Source: CDC
This image is in the public domain and thus free of any copyright restrictions. Use of this image does not constitute this blog’s endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention.
Diphtheria is caused by the non-sporing, non-encapsulated, and non-motile Gram positive bacillus Corynebacteriæ diphtheriæ and Corynebacteriæ ulcerans. Respiratory diphtheria presents with a pharyngitis that often will be associated with a visible greyish membrane and enlarged anterior cervical lymph nodes with perinodal swelling, causing the classic bullneck appearance. Laryngeal diphtheria can be associated with progressive hoarseness and stridor, while nasal diphtheria can present with uni- or bilateral discharge and crustiness. As diphtheria has become increasingly rare in the United Kingdom, thanks to a successful immunisation campaign, it’s not always instantly recognisable for clinicians.
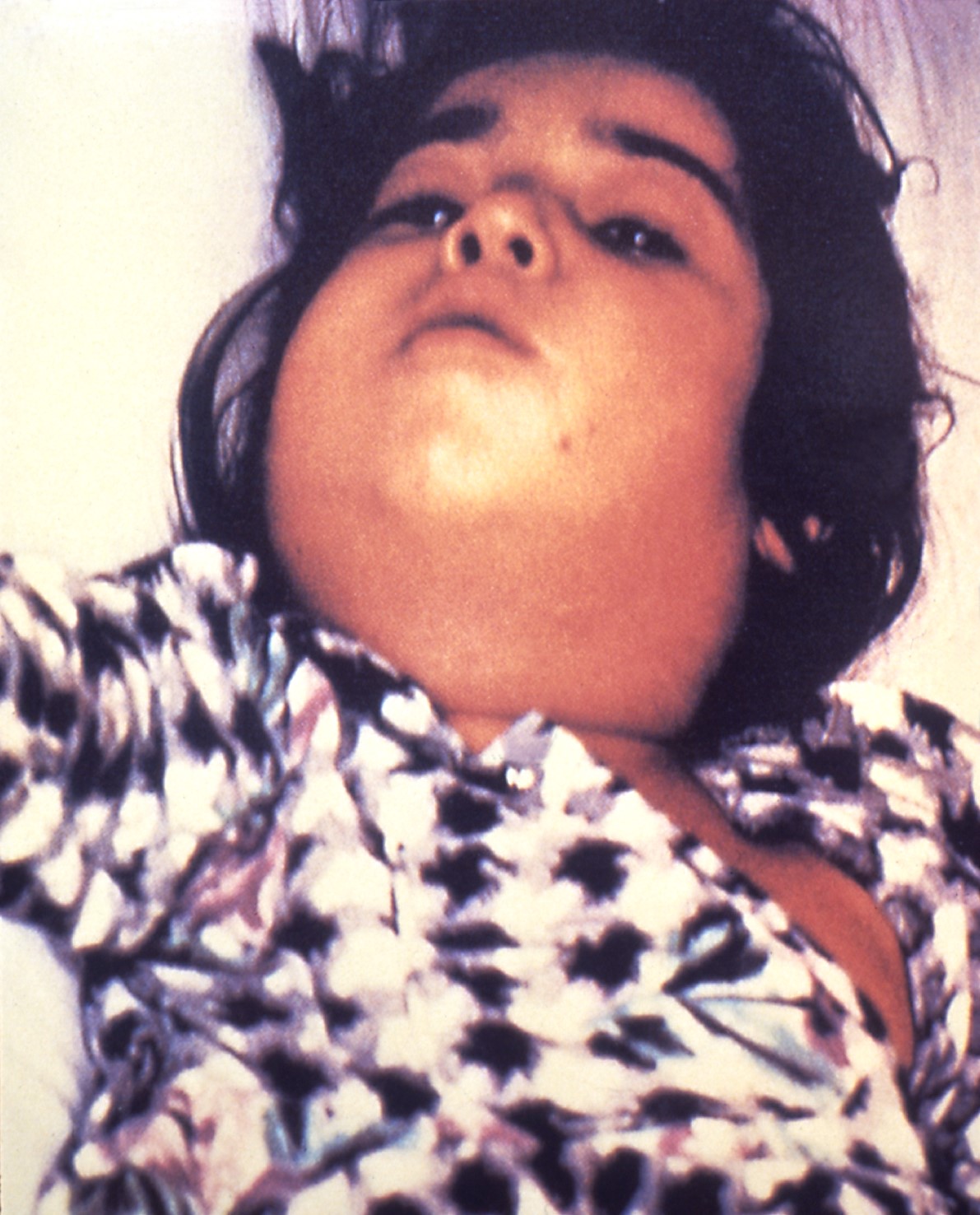 Figure 2 Bullneck appearance
Figure 2 Bullneck appearance
Source: CDC
This image is in the public domain and thus free of any copyright restrictions. Use of this image does not constitute this blog’s endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention.
The incubation period for diphtheria is usually 2 to 5 days but may be longer. The commonest mode of transmission of C. diphtheriæ is via droplet spread from a person with respiratory diphtheria; prolonged close contact is usually required for spread.
Cutaneous diphtheria usually starts as a vesicular rash and then forms one or more clearly demarcated ulcer that can be covered in a blueish-grey membrane. Patients may have both cutaneous and respiratory disease.
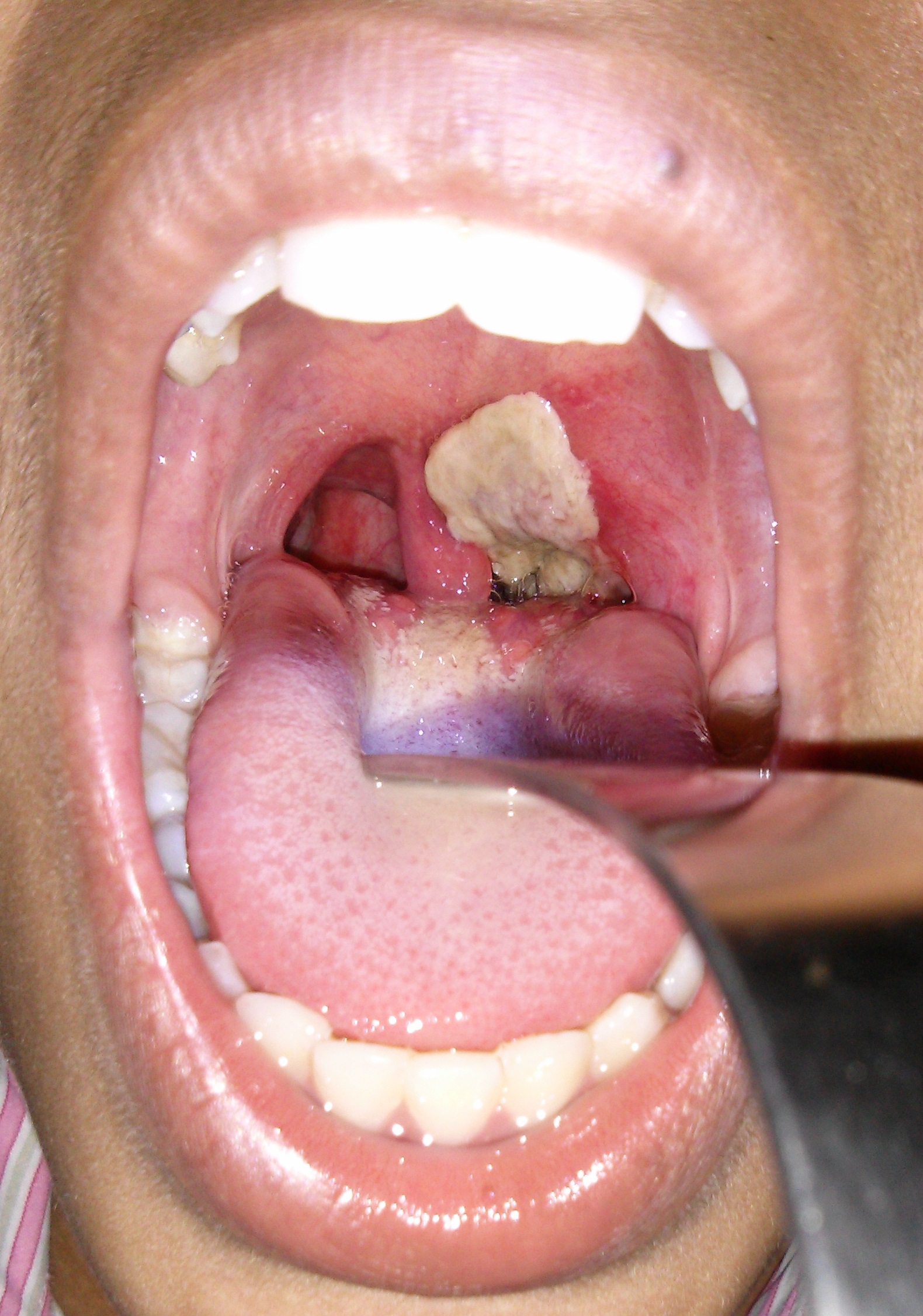
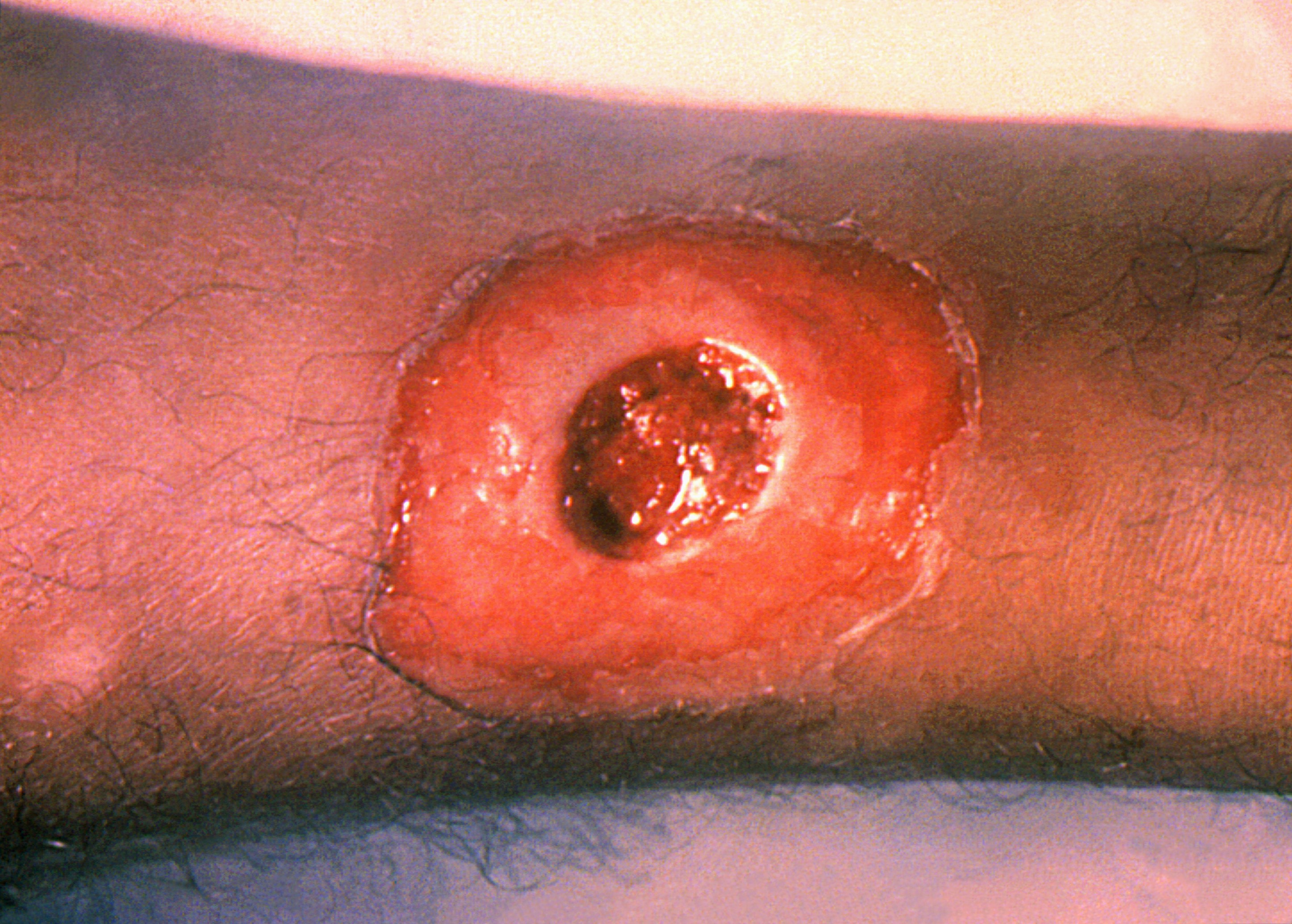
Figure 4 cutaneous diphtheria
Source: CDC
This image is in the public domain and thus free of any copyright restrictions. Use of this image does not constitute this blog’s endorsement or recommendation by the U.S. Government, Department of Health and Human Services, or Centers for Disease Control and Prevention.
Figure 3 respiratory diphtheria by User: Dileepunnikri is licenced under CC BY-SA 3.0 via Wikimedia Commons.
Swabs should be collected from patients and sent to a local diagnostic laboratory with appropriate clinical details.
All suspected cases have to be notified to the UK Health Security Agency. Patients might be referred to the local specialist infectious disease team for face-to-face assessment and review. Treatment options include diphtheria anti-toxin intravenously and macrolide antibiotics.
References
Knights, F; Munir, S (2022). Initial health assessments for newly arrived migrants, refugees, and asylum seekers. BMJ 2022;377:e068821 https://doi.org/10.1136/bmj-2021-068821
British Medical Association (2022). Unique health challenges for refugees and asylum seekers. https://www.bma.org.uk/advice-and-support/ethics/refugees-overseas-visitors-and-vulnerable-migrants/refugee-and-asylum-seeker-patient-health-toolkit/unique-health-challenges-for-refugees-and-asylum-seekers (Accessed 19.01.2023)
UK Health Security Agency (2022): Infectious diseases in asylum seekers: actions for health professionals. https://www.gov.uk/guidance/infectious-diseases-in-asylum-seekers-actions-for-health-professionals (Accessed 19.01.2023)
UK Health Security Agency (2022): Public health control and management of diphtheria in England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1117027/diphtheria-guidelines-2022_v17_111122.pdf (Accessed 19.01.2023)
 It is now over 40 years ago since a rare lung infection, Pneumocystis carinii was noticed in five young, previously healthy men who lived in Los Angeles1. The article describing this cluster or cases was the first hint of a global epidemic of human immunodeficiency virus (HIV), which at the time led inexorably to acquired immune deficiency syndrome (AIDS) and almost certain death.
It is now over 40 years ago since a rare lung infection, Pneumocystis carinii was noticed in five young, previously healthy men who lived in Los Angeles1. The article describing this cluster or cases was the first hint of a global epidemic of human immunodeficiency virus (HIV), which at the time led inexorably to acquired immune deficiency syndrome (AIDS) and almost certain death.
Fast forward to 2022 and HIV has become a treatable chronic disease – those who are adherent to antiretroviral therapy (ART) with an undetectable viral load can expect a normal or near-normal life span2 and no longer have to worry about passing the virus to a sexual partner, as this is impossible for those on effective treatment3.
The most important factor in reducing morbidity and mortality is early diagnosis and early initiation of ART. Progress of the virus is measured by monitoring the viral load and CD4 count (a type of T lymphocytes). If HIV is diagnosed late (when the CD4 count has dropped below 350 cells/mm3), there is a ten-fold increase in risk of the patient dying in the year after diagnosis4,5 and if this doesn’t happen, they still have a likely ten-year reduction in life expectancy2. It is estimated that 6,600 people in England have undiagnosed HIV, making up approximately 6% of the total population of people with HIV in England6. This group will have a poor prognosis if they remain undiagnosed, and risk unknowingly passing the virus to their sexual partners.
NICE guidance advises that we offer and recommend HIV testing based on local prevalence, which can be seen on this map of diagnosed HIV prevalence per 1,000 population aged 15 – 59 years in England in 2015. There are hotspots of high prevalence (2-5 cases per 1,000) and extremely high prevalence (>5 cases per 1,000) in various cities including London, Brighton and Manchester – healthcare professionals in these areas should be aware of the recommendation to offer routine HIV tests as laid out in the table below7, which also covers the clinical situations in which any healthcare professional should offer a test, regardless of local prevalence.
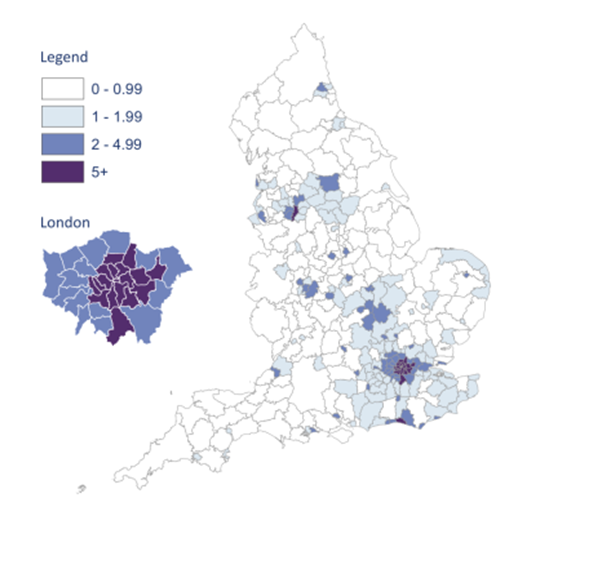
Map from HIV in the UK 2016 Report, Public Health England8
Image used under the Open Government Licence v3.0
|
Prevalence level |
When to offer a test |
|
High |
|
|
Extremely high |
|
|
Any prevalence |
|
In primary care we should be aware that HIV may present at seroconversion, or later as the CD4 count drops and symptoms of immunodeficiency start to emerge.
Seroconversion occurs between 10 days and six weeks after infection with HIV and may be very mild. It is difficult to diagnose as clinical features can be non-specific, including a fever, sore throat, malaise and arthralgia. A maculopapular rash, particularly in combination with a fever, should alert us to the possibility of seroconversion2. Traditionally we have considered there to be a 12 week ‘window period’ for HIV testing – the time in which a person may have contracted HIV, but still have a negative test as they have not yet made enough antibodies for the test to be positive. Many labs now use combined antibody/antigen tests which shorten the window period, but unless you are familiar with the tests used locally, it is safest to advise that anyone who may be in the early stages of HIV repeats their test at least 12 weeks after any encounter where they think they may have caught the virus.
Later symptoms are listed in the British HIV Association list of ‘clinical indicator diseases’, which are defined by the fact that patients with these conditions have at least a 1 in 1000 likelihood of having undiagnosed HIV9. They include diagnoses and incidental findings which are commonly seen in general practice, such as unexplained fever, lymphadenopathy, chronic diarrhoea or weight loss, unexplained low white cell count or platelet count and any cervical dysplasia or community acquired pneumonia. We should also consider testing for various skin conditions, including seborrhoeic dermatitis, shingles and severe psoriasis, and for those with lung cancer or lymphoma.
It is not all that often that we can point to a specific consultation in primary care and say “I saved a life today”. Picking up a case of HIV and getting a patient on to antiretrovirals is a truly life-saving measure, for which an increase in testing is vital.
References (all accessed 9.10.22)
1. HIV.gov. A timeline of HIV and AIDS. https://www.hiv.gov/hiv-basics/overview/history/hiv-and-aids-timeline#year-1981
2. NICE CKS. HIV infection and AIDS. 2021. https://cks.nice.org.uk/topics/hiv-infection-aids/
3. National Institute of Allergy and Infectious Diseases. HIV Undetectable=untransmissible, or Treatment as Prevention. 2019. https://www.niaid.nih.gov/diseases-conditions/treatment-prevention
4. The Lancet HIV. Time to tackle late diagnosis. Lancet HIV. 2022 Mar;9(3): e139
5. UK Health Security Agency. Leaving it late: why are people still dying from HIV in the UK? 2014.
6. Public Health England. Trends in HIV testing, new diagnoses and people receiving HIV-related care in the United Kingdom: data to the end of December 2019. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959330/hpr2020_hiv19.pdf
7. NICE NG60. HIV testing: increasing update among people who may have undiagnosed HIV. 2016. https://www.nice.org.uk/guidance/ng60/chapter/Recommendations#offering-and-recommending-hiv-testing-in-different-settings
8. Public Health England. HIV in the UK 2016 Report. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/602942/HIV_in_the_UK_report.pdf
9. British HIV Association. British HIV Association/British Association for Sexual Health and HIV/British Infection Association Adult HIV Testing Guidelines 2020. 2020. Appendix 1. https://www.bhiva.org/file/5f68c0dd7aefb/HIV-testing-guidelines-2020.pdf
 Introduction
Introduction
Constipation is a common, frequently encountered gastrointestinal disorder affecting approximately one third of adults 60 years and older, with over half of nursing home residents affected. Constipation can have significant consequences: in susceptible and frail older people, excessive straining can trigger a syncopal episode and coronary or cerebral ischaemia. In severe cases, constipation can lead to anorexia, nausea and pain and ultimately can cause a stercoral ulcer, leading to perforation and death. Stercoral ulcers are due to hard, impacted faeces causing pressure necrosis of the distal colon, leading to ulceration and subsequent perforation of the intestinal wall.
Constipation often causes a reduction in the quality of life, with general health, vitality, social functioning and mental health all affected. Risk factors for constipation include female sex, older age, inactivity, low calorie intake, a low-fibre diet, polypharmacy, and low income. The incidence of constipation is three times higher in women, and women are twice as likely as men to see their primary care team for constipation.
Symptoms
Patients usually complain of ‘straining’ when opening their bowels, difficulty passing stools, incomplete evacuation or both. This is often associated with hard stools, abdominal bloating pain and distention. The stool frequency may be normal.
Primary constipation
Primary constipation includes the subtypes of normal transit, slow transit and disorders of defaecation, all of unknown causes. Histology of the colon of older adults shows more tightly packed collagen fibres and a reduced number of myenteric neurons, but these changes are not considered to be major contributors to the development of constipation.
Secondary constipation
Secondary causes of constipation include medication use, chronic disease processes and psychosocial issues. Opioids, calcium channel blockers, oral iron supplements, antacids and anticholinergics are common causes for medication-induced constipation, while hypothyroidism, hypercalcaemia, Parkinson’s disease and colorectal carcinoma can all cause secondary constipation.
Assessment
Patients should always be assessed for red flags that might indicate an underlying malignancy, such as:
· persistent unexplained change in bowel habits
· palpable mass in the lower right abdomen or the pelvis
· persistent rectal bleeding without anal symptoms
· narrowing of stool calibre
· family history of colon cancer, or inflammatory bowel disease
· unexplained weight loss, iron deficiency anaemia, fever, or nocturnal symptoms
· severe, persistent constipation that is unresponsive to treatment.
NICE guidance NG12 gives primary care practitioners a clear framework when to refer a patient with suspected colorectal cancer on a two week wait (2ww) suspected cancer pathway referral.
When evaluating an older individual with constipation, taking a thorough drug and medical history and performing a physical examination are important. Abdominal examination might reveal abnormal bowel sounds, significant weight loss, cachexia, masses and/or sigmoid or colonic loading. When planning a rectal examination, the patient should be informed about its diagnostic importance and the fact that it might be uncomfortable. A chaperone should always be offered and consent documented. Examination of the anus might reveal abnormalities such as proctitis, haemorrhoids, prolapse, fissures or rectal cancer, while further up in the ampulla a rectal-digital examination can detect faecal impaction, masses, and gives the examiner the chance to evaluate anal tone.
Management
Non-pharmacological treatment is often the first step to help patients in the management of their complaint with adaptation of the patients’ medication a start to reduce symptoms.
Endocrinological/psychological/neurological causes of secondary constipation will need investigation and appropriate management. It might be worth discussing with the patient that there is no physiological necessity to have a daily bowel motion: a discussion around simple lifestyle changes might improve their perception of bowel regularity and a diary reporting on stool pattern and consistency may be helpful as well. The optimal time to have a bowel movement is often after waking and/or after meals, when the colon’s motor activity is particularly pronounced. A gradual increase in the intake of fluids and fibre should be suggested, but we should be careful in patients with cardiorenal issues not to cause overloading. A prospective study from Japan found that placing the patient’s feet on a small foot stool in front of the toilet, in conjunction with the upper body bent forward, improved anal pressure and reduced time of evacuation. Patients in care homes should be given adequate time and privacy for their bowel movements and should avoid bed pans.
Most patients will, at one stage, require a laxative to alleviate their symptoms when lifestyle interventions are ineffective: in patients with short-duration constipation a bulk forming laxative such as ispaghula husk should be initiated. If these are not helpful, an osmotic laxative such as macrogol should be next.
In chronic constipation, a bulk forming laxative should be initiated, with adequate hydration ensured (low fluid intake with bulk forming laxatives can cause impaction). If defaecation continues to be unsatisfactory, an osmotic laxative should be the next choice, with the addition of a stimulant laxative such as bisacodyl if the patient doesn’t improve. Once regular bowel movements occur, the laxatives can be slowly withdrawn.
If there is no response to the maximum tolerated dose of second line laxatives, refer to the local GI or older people’s outpatient team, or ask for guidance via the local advice and guidance process.
If the patient presents with faecal impaction, the appropriate escalating dose of a macrogol should be considered. In those with soft stools, or with hard stools after a few days’ treatment with a macrogol, an oral stimulant laxative should be started or added to the previous treatment. If the response continues to be underwhelming, rectal administration of bisacodyl (for soft stools) or glycerol (for hard stools) can be considered.
If there is still no response, a sodium acid phosphate with sodium phosphate enema may have to be considered. For hard stools, an overnight arachis oil enema, followed by a sodium acid phosphate with sodium phosphate enema the next morning might prove effective.
In patients with opioid-induced constipation, an osmotic laxative (or docusate sodium to soften the stools) and a stimulant laxative is recommended. Bulk-forming laxatives should be avoided.
If the patient’s presentation is getting worse or there is no response, then consult with one of your colleagues from gastroenterology. Further input from these teams might be needed for additional pharmacotherapy, endoscopy, anorectal manometry or other secondary/tertiary care investigations.
References:
Arco, S; Saldaña, E et al (2021). Functional Constipation in Older Adults: Prevalence, Clinical Symptoms and Subtypes, Association with Frailty, and Impact on Quality of Life. Gerontology DOI: 10.1159/000517212
British National Formulary: Constipation https://bnf.nice.org.uk/treatment-summaries/constipation/ accessed 14/7/2022
Gandell, D; Straus, SE et al (2013). Treatment of constipation in older people. Canadian Medical Association Journal, May 14, 185(8) DOI:10.1503/cmaj.120819
De Giorgio, R; Ruggeri, E et al (2015). Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterology 15:130 DOI 10.1186/s12876-015-0366-3
Gough, AE; Donovan, MN et al (2016). Perforated Stercoral Ulcer: A 10-year experience. Journal of the American Geriatrics Society. Vol 64, No 4
Heidelbaugh, J; Martinez de Andino, N et al (2021). Diagnosing Constipation Spectrum Disorders in a Primary Care Setting. Journal of Clinical Medicine. (10) 1092. https://doi.org/10.3390/jcm10051092
Hull and East Riding Prescribing Committee (2019). Management of Constipation in Adults. https://www.hey.nhs.uk/wp/wp-content/uploads/2019/08/GUIDELINE-Constipation-guidelines-updated-may-19.pdf accessed 14/7/2022
Jamshed, N; Lee, Z; Olden, KW (2011). Diagnostic Approach to Chronic Constipation in Adults. American Family Physician. 84(3):299-306
Mounsey, A; Raleigh, M et al (2015). Management of Constipation in Older Adults. American Family Physician Volume 92, Number 6
National Institute for Health and Care Excellence (2021). NG12 Suspected cancer: recognition and referral. https://www.nice.org.uk/guidance/ng12/chapter/Recommendations-organised-by-site-of-cancer#lower-gastrointestinal-tract-cancers accessed 14/7/2022
Rome Foundation (2016) Appendix A: Rome IV Diagnostic Criteria for FGIDs. https://theromefoundation.org/rome-iv/rome-iv-criteria/ accessed 14/7/2022
Schuster, BG; Kosar, L et al (2015). Constipation in older adults; Stepwise approach to keep things moving. Canadian Family Physician 61: February
Takano, S; Nakashma, M et al (2018). Influence of foot stool on defecation: a prospective study. Pelviperineology 2018; 37: 101-103
Villanueva Herrero JA, Abdussalam, A et al (2022). Rectal Exam. In StatPearls. StatPearls Publishing. https://pubmed.ncbi.nlm.nih.gov/30726041/
 July 2022 will see Integrated Care Systems (ICS) become statutory in England. Partnerships between NHS service providers, commissioners, local authorities, and other organisations will be responsible for planning, co-ordinating and commissioning health and care services that meet the needs of each geographically defined community. Included within each community will be a relatively small number of people who are in contact with the criminal justice system, some of whom will go in and out of prison more than once. It is recognised that this cohort, while heterogeneous, often has highly complex needs and frequently experiences poorer than average health access, experience, and outcomes.
July 2022 will see Integrated Care Systems (ICS) become statutory in England. Partnerships between NHS service providers, commissioners, local authorities, and other organisations will be responsible for planning, co-ordinating and commissioning health and care services that meet the needs of each geographically defined community. Included within each community will be a relatively small number of people who are in contact with the criminal justice system, some of whom will go in and out of prison more than once. It is recognised that this cohort, while heterogeneous, often has highly complex needs and frequently experiences poorer than average health access, experience, and outcomes.
Whilst in prison, men and women from this cohort will be temporarily ‘hidden’ from their community ICS commissioning landscape (commissioning of prison healthcare takes place separately, by NHS England and NHS Improvement health justice commissioners in England, Health Boards in Wales and Scotland and the South East Trust in Northern Ireland), and may be in secure settings distant from the locality to which they will return. It is essential that, as integrated care systems are launched, there will be representation and acknowledgement of the needs of these patients to avoid compounding the health inequalities gap. NHS England and NHS Improvement have introduced Core20PLUS5, an approach to address health inequalities at national and system levels. It identifies the most deprived 20% of the population, plus those not captured in the 20% who experience poorer than average health and recognises five ‘focus’ clinical areas requiring accelerated improvement. Those in touch with the criminal justice system are considered an ‘inclusion’ group in the PLUS cohort. This strategy aims to ensure that health and care needs are met in people in contact with the criminal justice system.
Once in prison, people are not able to choose where or from whom they receive their healthcare provision. Healthcare services in secure environments need to be configured to take account of the particular needs of the population they are serving, for example with regard to the increased prevalence of mental ill health, substance misuse and communicable diseases, whilst also acknowledging the distinctive settings in which the care needs to be delivered.
Healthcare practitioners have a duty of care to their patients, and in the secure setting this duty must be delivered in the context of the physical environment and lifestyle constraints prison brings, whilst utilising the assistance of the staff providing the security for the establishment. As a result, healthcare delivery in the secure setting requires continual collaboration and partnership working between prison and healthcare staff to deliver the most beneficial services.
A healthcare worker in a prison has a unique opportunity to address the needs of some of society’s most vulnerable people and this must be done without prejudice. This means that the nature of someone’s offence, or the reason for their detention, should not alter how patients are cared for.
Particular risks to patient safety, engagement and health equity occur at transition points, as people move into and out of prison, or are moved from one prison to another. NHS RECONNECT schemes have been set up to bridge the gap for patients being released from prison. They aim to facilitate continuity of care by ensuring that health information is shared, and connections are built with community health care practitioners before a patient leaves prison. The success of pre-release healthcare arrangements requires careful coordination with the probation service and the local authority to ensure that suitable housing is identified within the locality that community healthcare provision has been arranged.
In 1996, Her Majesty’s Chief Inspector of Prisons, Sir David Ramsbotham, published his paper “Patient or Prisoner?” in which his terms of reference were: ‘to consider health care arrangements in Prison Service establishments in England and Wales with a view to ensuring that prisoners are given access to the same quality and range of health care services as the general public receives from the National Health Service’. This paper introduced the concept of ‘equivalence’ of care and set the scene for what continues to be an evolving area of prison and secure environment medicine.
The principle of equivalence has been instrumental in helping to define and contrast the care being delivered in secure settings with that of the care in the wider community, with the aim of mirroring of provision. Since 2006 and the move towards the commissioning of health services in prisons by the National Health Service, there has been a significant transformation in the quality and consistency of services being delivered to people in prison. By aiming to deliver healthcare services that are ‘equivalent’, and achieving equitable health outcomes, we are not only striving to improve the health of our secure and detained patients, we are also benefitting society as a whole.
The RCGP has recently launched Secure Environments Hub that features information about healthcare in secure environments and eLearning for clinicians and multi-disciplinary team related to the prison system. You can access the Hub here: https://elearning.rcgp.org.uk/course/view.php?id=561
The majority of people, including health care professionals (HCPs), have unconscious or implicit biases1. These can affect the way we interact with colleagues, staff and patients, and even influence our decision making in regards to diagnosis and management of conditions2.
Unconscious bias is the immediate judgement of something, or someone, based on our past experience, background and culture. It is instinctive rather than a rational thought process and occurs almost instantaneously on encountering someone new. This bias can then influence our thoughts, beliefs and behaviour towards that person.
When we first meet someone, we subconsciously categorise them based on, for example, their gender, age, skin colour, accent, profession, sexual orientation3. We then use preconceived ideas of their intrinsic characteristics and form an immediate opinion about the person. Most people have unconscious biases, no matter how strongly they consciously oppose discrimination or prejudice. We tend to feel positively about someone who is similar to us, and negatively about those we perceive as ‘different’. These assumptions then effect the relationship we have with that person, including how close we will stand to them, and how often we make eye contact2.
The term unconscious bias encompasses several types of bias such as gender bias, confirmation bias, age bias, and affinity bias, to name a few. These biases can affect everything from to which candidate we would offer a practice vacancy, to how we manage different patients with the same condition. It can be both positive and negative, as we may favour or disapprove of someone based on whether we feel they fit into ‘our group’ (namely, whether we feel they share similar characteristics to us).
Take a vacant salaried GP position as an example: You’re on the interview panel. The next applicant trained at the same medical school and enjoys the same hobbies as you. You immediately think ‘yes, this person is great’. The next applicant attended a rival medical school and has different hobbies; you’re not so sure about this applicant. This is an example of affinity bias. These opinions are formed without us even realising it and without taking into account the person’s qualifications or experience. Once that initial judgement is formed, our brain then begins to gather evidence to support our assessment. However, this ‘evidence’ is also biased (confirmation bias) as we look for anything that will uphold our initial decision and disregard items that refute it.
Unconscious bias is well recognised in the interviewing process, and many private companies have procedures in place to reduce the risk of this happening4. But how does this translate into the world of healthcare?
Unconscious bias may have a significant impact on medical school admissions, with one American study reporting a notable race bias5 by those on the selection panel. International medical graduates (IMGs) are up to thirteen times more likely to be referred to the GMC than UK graduates6, which is thought to be due, at least in part, to unconscious bias. We commonly hear of female doctors being called nurse whilst male nurses or medical students are called doctor and are often talked to in preference to their senior female colleague.Not only does unconscious bias affect us and our colleagues, but it also has a crucial and concerning impact on our patients. In 2021 Mothers and Babies: Reducing Risks through Audits and Confidential Enquiries across the UK (MBRRACE-UK) released a report7 that showed black women were four times more likely to die in pregnancy than white women. Whilst socioeconomic factors and medical reasons were thought to contribute to the outcomes, this only accounted for a small proportion of women. Black women report not being listened to or empathised with as much as their white counterparts8.
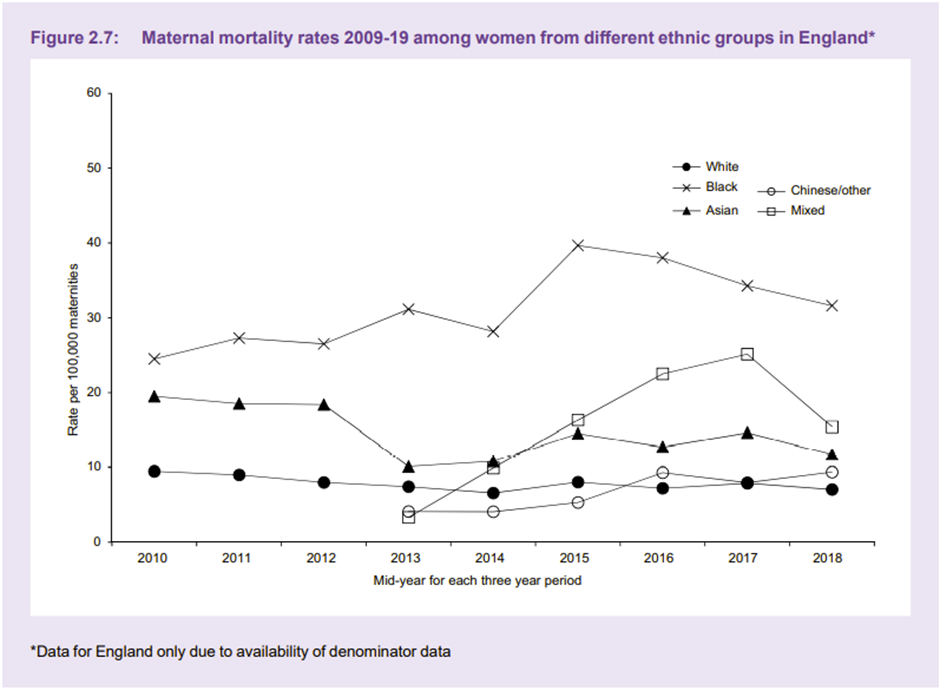
Graph from MMBRACE-UK 'Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017-19'. Used with permission from MMBRACE-UK.
Several studies have shown that there is racial bias in regards to pain management, with black people frequently denied analgesia that their white counterparts are readily offered. Research by Hoffman et al (2016) reported that the false belief of biological differences between white and black people contributing to pain thresholds, are held by the general population, and more worryingly by medical students and qualified doctors9. This not only impacts women in labour, but all situations where adequate pain relief is important.
The Midlands Leadership Academy has developed an Unconscious Bias Toolkit10 which suggests several ways in which we can challenge our own unconscious biases. The toolkit advises us to consider:
- What am I thinking?
- Why am I thinking it?
- Is there a past experience that is impacting my current decision?
- Is the past experience applicable now or is it based on a preference or bias?
Furthermore, the Royal Society4 advise that we should slow down when making decisions, reconsider reasons for decisions, question cultural stereotypes and monitor each other for unconscious bias. The Royal College of Surgeons have published a document on reducing unconscious bias11 in which they suggest using Thiederman’s Seven Steps for defeating bias in the workplace.
|
1. Become mindful of your biases 2. Put your biases through triage 3. Identify the secondary gains of your biases 4. Dissect your biases 5. Identify common kinship groups 6. Shove your biases aside 7. Fake it till you make it (what we say can become what we believe) |
Medical education - whether undergraduate or postgraduate - has a responsibility to promote curricula in a non-biased way. There has recently been a push to decolonise medical education and incorporate cultural safety12: Decolonising medical education involves challenging beliefs and introducing ‘new normals’ such as representing signs and symptoms of illnesses in different skin tones; or promoting issues faced by discriminated groups, for example, including violence against women and racism in curricula13. Cultural safety is a concept developed by Māori Nurse Educator Irihapeti Merenia Ramsden, who recognised the health inequalities between indigenous and non-indigenous people in New Zealand. Cultural safety is to understand that health inequality is based on historical prejudice, and using lived experiences of those who have experienced discrimination ensures that differences in culture are respected throughout healthcare. If health care professionals and students understand how conditions may present in different ethnicities and genders, really listen to patients experiences and concerns regardless of their skin colour or own beliefs, and learn to challenge their unconscious bias, then hopefully we will develop a generation of healthcare professionals who can more fully appreciate and challenge health inequality in the UK.
The RCGP has recently launched an interactive eLearning module on Allyship, which includes information and suggested actions to promote anti-racism and bystander intervention. You can access the course here: https://elearning.rcgp.org.uk/allyship
References
- Schwarz, J., 1998. Roots of unconscious prejudice affect 90 to 95 percent of people, psychologists demonstrate at press conference, University of Washington, [online]. Available at: https://www.washington.edu/news/1998/09/29/roots-of-unconscious-prejudice-affect-90-to-95-percent-of-people-psychologists-demonstrate-at-press-conference/ [Accessed 06 April 2022]
- FitzGerald, C. and Hurst, S., 2017. Implicit bias in healthcare professionals: a systematic review. BMC Medical Ethics, 18, 19. https://doi.org/10.1186/s12910-017-0179-8.
- Rice, M., 2015. Unconscious bias and its effect on healthcare leadership. Healthcare Network, The Guardian. [online] Available at: https://www.theguardian.com/healthcare-network/2015/may/19/healthcare-leadership-best-practice-unconscious-bias. [Accessed 04 April 2022].
- Frith, U., 2015. Understanding Unconscious Bias. The Royal Society. [online] Available at: https://royalsociety.org/topics-policy/publications/2015/unconscious-bias/?gclid=CjwKCAiA6seQBhAfEiwAvPqu18TssncdweUmNPHBiUb8o2UHBslILV8UOvM-fs7ePvbngXtmq6fdrRoC-PYQAvD_BwE. [Accessed 04 April 2022].
- Capers, Q., 4th, Clinchot, D., McDougle, L., and Greenwald, A. G., 2017. Implicit Racial Bias in Medical School Admissions. Academic Medicine: Journal of the Association of American Medical Colleges, 92(3) pp.365–369. https://doi.org/10.1097/ACM.0000000000001388
- Rimmer A, 2017. Unconscious bias must be tackled to reduce worry about overseas trained doctors, says BAPIO. British Medical Journal, 357 :j1881 doi:10.1136/bmj.j1881.
- Knight, M., et al on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017-19. [online] Oxford: National Perinatal Epidemiology Unit, University of Oxford 2021. Available at: https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2021/MBRRACE-UK_Maternal_Report_2021_-_FINAL_-_WEB_VERSION.pdf [Accessed 04 April 2022]
- Brathwaite, C., 2018. Black Mothers Are Disproportionately More Likely To Die In Childbirth - We Need To Address The Race Gap In Motherhood. Huffington Post [online] Available at: https://www.huffingtonpost.co.uk/entry/race-motherhood-black-mothers-dying-childbirth_uk_5c078da3e4b0a6e4ebd9d5ec [Accessed 04 April 2022].
- Hoffman, K.M., Trawalter, S., Axt, J.R., and Oliver, M.N., 2016. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences of the United States of America, 113(16), pp.4296–4301. https://doi.org/10.1073/pnas.1516047113.
- Masuwa, P. and Sharma, M. Unconscious bias toolkit. NHS Midlands Leadership Academy. [online] Available at: https://midlands.leadershipacademy.nhs.uk/wp-content/uploads/sites/3/2020/12/Unconscious-bias-toolkit-final-version.pdf.
- Royal College of Surgeons of England, 2016. Avoiding Unconscious Bias: A guide for surgeons. [online] London: The Royal College of Surgeons of England (Published 2016) Available at: https://www.rcseng.ac.uk/library-and-publications/rcs-publications/docs/avoiding-unconscious-bias/ [Accessed 04 April 2022]
- Wong, S.H.M., Gishen, F. and Lokugamage, A.U., 2021. ‘Decolonising the Medical Curriculum‘: Humanising medicine through epistemic pluralism, cultural safety and critical consciousness. London Review of Education, 19(1). DOI: 10.14324/LRE.19.1.16.
- Lokugamage, A.U., Ahillan, T. and Pathberiya, S.D.C., 2020. Decolonising ideas of healing in medical education. Journal of Medical Ethics 46(4), pp.265-272. http://dx.doi.org/10.1136/medethics-2019-105866.
Children with life-limiting conditions face significant challenges when they transition from paediatric to adult care. The transition involves many changes: the young person will transfer to adult specialities, to adult social care, and will experience changes in educational provision and legal changes involving parental responsibility for medical decision making.
The transition has been described as a ‘cliff-edge’ event, during which children and their families face significant stress, challenges, and unmet needs. When general practice is already aware of such a patient within their practice, a good service is already provided in many cases, but often young people with these conditions are not always visible, as the majority of their care is provided by paediatrics. Many of these young patients who thus far have had long term continuity of care from their paediatricians feel nervous or have little confidence in general practice and their ability to meet their complex needs. In some cases this can lead to ‘fire-fighting’, where general practice has to respond to crisis calls from young people (or their carers) with whom they may have had limited contact.
Recently, a small team at the RCGP – together with their colleagues from ‘Together for Short Lives’, produced an eLearning course on how the whole primary care team can contribute towards a good health transition from paediatric to adult care. These modules are for all primary care clinicians who want to provide excellent care for a young person with a chronic and/or life-limiting condition who is transitioning from paediatric to adult care. The modules are based on the insights generated by a pilot project which involved a steering group inclusive of general practice, palliative care, paediatricians, academics, and ‘Together for Short Lives’. Written by Dr Mike Miller, a paediatric palliative consultant with first hand experience of the pitfalls during transition and Dr Peter Lindsay, a GP with a special interest in paediatrics, the course combines the latest academic insights on health transition and the experiences of the authors and the steering group team.
During the pilot project, we recognised that there is overlap between young people with chronic and/or life-limiting conditions and young people with learning disabilities, and although the groups are distinct, there are similarities in their transition process. In many areas, local transition services for young people with complex needs are evolving rapidly: Leeds have developed an evolving transition network, and so far involves ongoing collaborations between education, the local children's hospice, social care, mental health services and learning disability leads.
As part of the pilot project, we performed initial searches within our practices for patients within these categories, and are now trying to develop processes to support transition. The pilot project highlighted the positive impact we can have in supporting the young person and family at a challenging and stressful time, and how we can improve health and social outcomes. As well as the routine care offered, general practice is uniquely placed to offer continuity of care through the transition period, early preparation for the transition to adult service, social prescribing and the help of the whole primary care team. Primary care is also perfectly placed to address health needs that often go under-recognised in this group, such as sexual health and contraception. If we can develop mechanisms to reliably identify these patients, then young people with chronic and/or limiting conditions will benefit from all that primary care can offer.
The eLearning modules firstly outline the challenges facing young people and their families during the transition period, from the perspective of a paediatric palliative care consultant who has devoted much of his career to improving the experience of young people and their families at transition. Secondly, poorly understood areas are tackled, such as the legal framework underpinning mental capacity at transition, and the practical implications of this for the young person and family. The second module presents practical approaches and best practice to support this area in a GP setting, in the current challenging and busy context of general practice. Please see below for some tasters from the eLearning modules.
Please also see the Developing Positive Transitions for Primary Care resources section, which signposts to further key information.
If you would like to learn more please see our eLearning modules and further learning resources:
'Better transitions: improving young people’s transfer from paediatric to adult services' eLearning course: https://elearning.rcgp.org.uk/bettertransitions
'Developing Positive Transitions for Primary Care' resources page: https://elearning.rcgp.org.uk/course/info.php?id=329